Vesical fissure in a newborn female: A variant of bladder exstrophy
During a newborn's initial examination, doctors discovered a non-erythematous, midline, suprapubic dimple located 5 cm below the level of the umbilicus. The remainder of the examination was unremarkable. On the second day of life, the newborn had a wet diaper with urine appearing to originate from 2 separate sources, including the dimple. What's the diagnosis?
The case
A 3720g (75th percentile) female infant was born at 40 weeks and 3 days gestation to a 30-year-old female following and uncomplicated pregnancy with regular prenatal care. Her length and head circumference were measured at 50 cm (60th percentile) and 35 cm (75th percentile), respectively. Her initial newborn examination revealed a non-erythematous, midline, suprapubic dimple located 5 cm below the level of the umbilicus (Figures 1, 2). The remainder of her examination was unremarkable, including head/scalp, eyes, ears, heart, back, umbilicus, anus, and genitalia. She had no hepatosplenomegaly and no skin lesions.
Figure 1
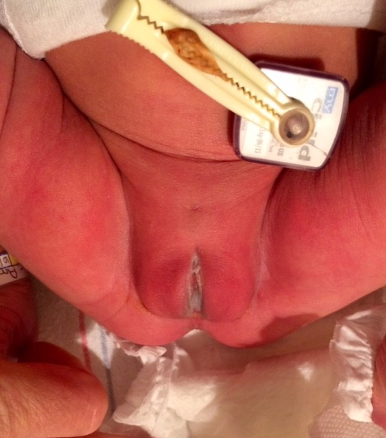
Figure 2
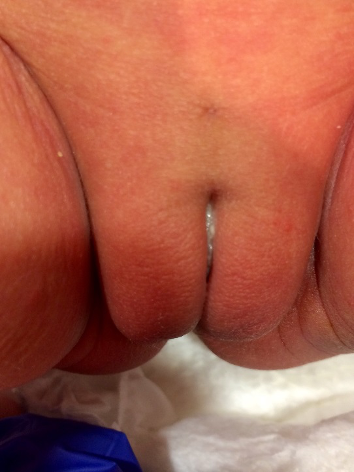
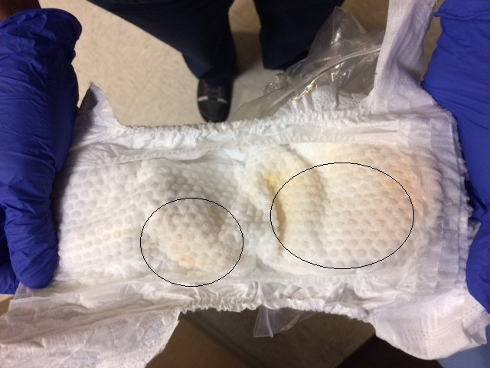
On the day of birth, white discharge was removed from this dimple. On the second day of life, she had a wet diaper with urine appearing to originate from 2 separate sources, including the dimple (Figure 3). However, using gentle manual palpation, examiners were unable to express urine through the dimple. Patient had 2 wet diapers in the first day of life and 4 wet diapers on the second day of life. She had 4 stools in the first 48 hours of life.
Figure 3
Evaluation and testing
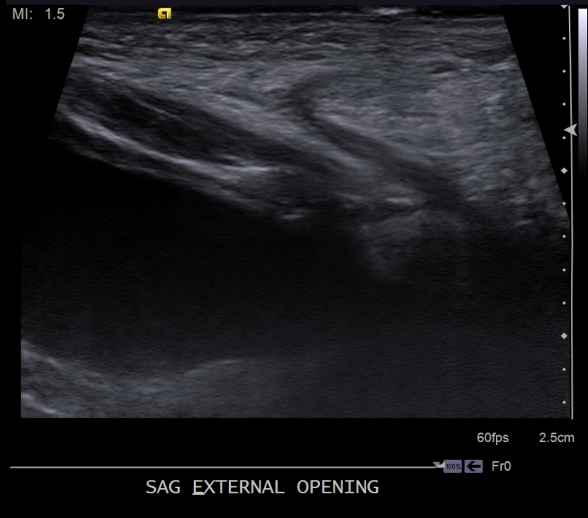
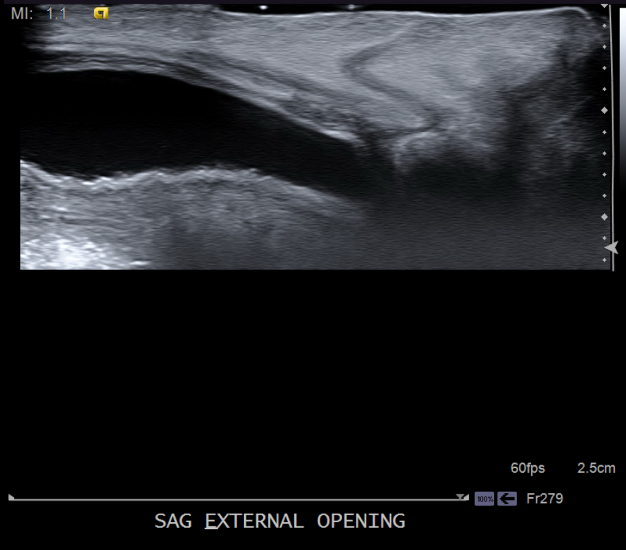
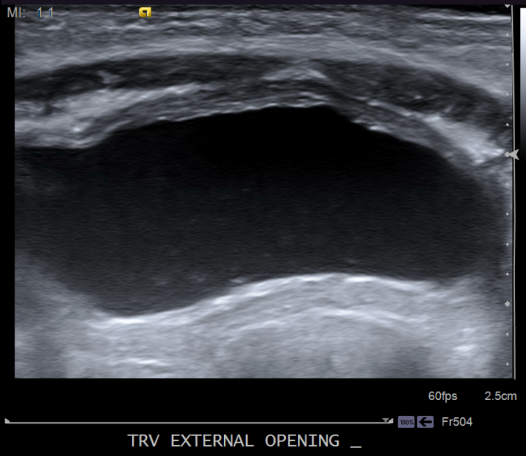
Our patient underwent abdominal ultrasound, which noted a fluid-filled tract from the lower anterior bladder to the suprapubic lower anterior abdominal wall, measuring approximately 2 mm in thickness/diameter and appearing to communicate with the bladder (Figures 4-7). The bladder wall was mildly and diffusely thickened. Renal sonography revealed normal kidneys without evidence of hydronephrosis, calculi, or masses. The remainder of the bladder appeared normal. Urology was consulted and recommended outpatient follow up for a voiding cystourethrogram (VCUG) and eventual surgical excision of the tract. No laboratory tests were recommended by the urologist given the normal renal ultrasonography and the normal urination. She was placed on amoxicillin at 10 mg/kg/day for urinary tract (UTI) prophylaxis, as the tract was in communication with the bladder and the bladder was already showing signs of wall thickening.
Figure 4
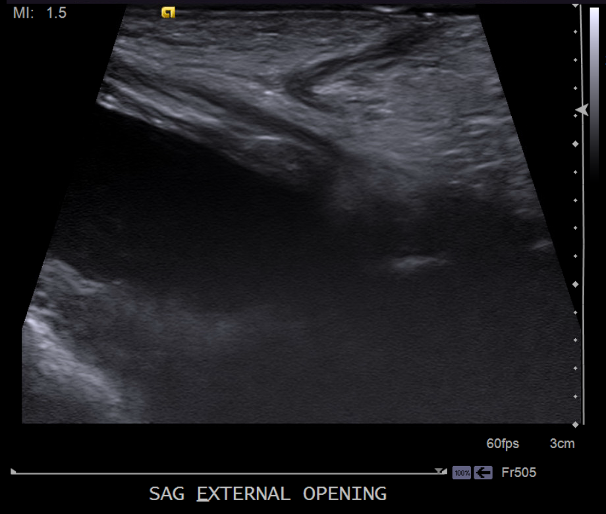
Figure 5
Note: Needle puncture during fetal life as a complication of diagnostic amniocentesis can result in skin dimpling that can occur anywhere on the body, in which case obtaining history of amniocentesis and ruling out other cases is key to diagnosis.
Figure 6
Every newborn should be inspected for any abnormalities, some of which may be benign and/or transient. Some abnormalities may point to an underlying pathology that needs further evaluation.
Figure 7
Differential diagnosis
Pre-auricular pits are small indentations located anterior to the helix and superior to the tragus of the ear. They are present bilaterally in 25% to 50% of cases. They are common and occur in approximately 1% of white, 5% of black, and 10% of Asian children. Associated congenital anomalies occur in approximately one-third of the sporadic cases. In approximately one-third of the sporadic cases. Renal ultrasound should be performed in patients with isolated pre-auricular pits or in patients with other ear anomalies accompanied by 1 or more of the following: other malformations or dysmorphic features; a family history of deafness; auricular and/or renal malformations; or a maternal history of gestational diabetes. Our patient did not have any pre-auricular pits or auricular malformation.
Branchial cleft cysts appear as a small opening, skin tag, or dimpling in the upper part of the neck anterior to the sternocleidomastoid. Branchial cleft cysts are remnants of embryonic development and result from a failure to obliterate one of the branchial clefts. First branchial cleft cysts are usually found near the auricle; second branchial cleft cysts are found just below the angle of the mandible and anterior to the sternocleidomastoid muscle (SCM). The third branchial cleft cysts are also found anterior to the SCM but lower in the neck as compared to the second branchial cleft cysts. Patient did not have any dimples near the auricle, at the angle of the jaw, or in her neck.
Table
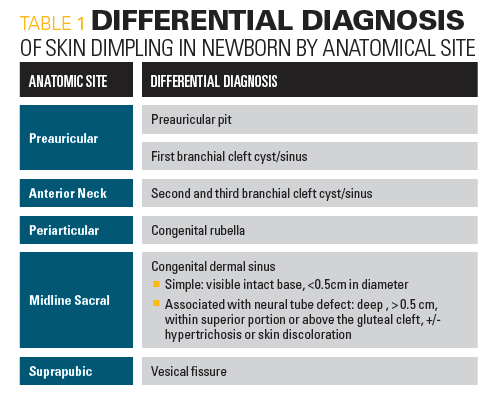
Congenital rubella associated dimples are skin dimples in congenital rubella that generally occur over each patella, the radial styloid process, the lower end of the ulna at each wrist, the bony medial and lateral epicondyles at each elbow, and/or 1 over each superior and inferior posterior iliac spine.1 Our patient did not have skin dimpling over any bony prominence and had no other findings to suggest intrauterine rubella infection. She did not have microcephaly, hepatosplenomegaly, purpura, or a heart murmur.
Midline skin dimpling in the back can be seen in congenital derma sinus tracts or diastematomyella (2 hemicords due to a congenital longitudinal split in spinal cord from an osseous or fibrous band). Small sacral dimples that overlie the coccyx and have well-visualized intact bases are considered benign variants. Dimples associated with excess hair, pigmentation, skin tags, or vascular anomalies need further ultrasound or magnetic resonance imaging to check for neural tube defects. Our patient did not have any midline back dimpling.
Skin dimpling may occur secondary to injury during amniocentesis.2 Amniocentesis is generally performed under ultrasonography during the second trimester when the ratio of viable to nonviable cells in the amniotic fluid is greatest. It is usually done on older pregnant women who are at greatest risk for delivering babies with genetic disorders. Our patient’s mother was 30 years old and did not undergo amniocentesis.
Discussion
Our patient had a midline suprapubic dimple but the rest of her physical exam was normal. She did not have any other physical findings suggestive of congenital rubella as her dimpling was located midline on her lower abdomen and not over patella, near the radius, ulna, elbow, or iliac spine. The mother’s history and laboratory tests did not indicate an intrauterine rubella infection. Our patient did not have any midline back dimpling to suggest congenital dermal sinus tract or underlying neural tube defect. Also, our patient did not have any periauricular, below the angle of the mandible, or anterior neck dimpling to suggest branchial cleft cysts. Evidence of urine seeping out of the pit pointed to a diagnosis of vesical fissures, confirmed with abdominal ultrasonography.
Diagnosis of vesical fissure
Bladder exstrophy occurs when normal embryological abdominal wall development fails.3,4 In this anomaly, there is a failure of the infraumbilical mesenchyme to migrate caudally and invade between the endoderm and ectoderm of cloacal membrane. As a result, the cloacal membrane becomes unstable and prone to rupture. Rupture of the cloacal membrane after the gastrointestinal tract and genitourinary tracts separate is a defining embryological etiology event in bladder exstrophy.5 Patients classically present with bladder mucosa that is open and exposed in the midline of the abdominal wall. The incidence for bladder exstrophy ranges from 2 to 4 per 100,000 live births.5 This condition is often diagnosed during prenatal ultrasound and can be associated with other abnormalities in the development of the bladder, abdominal wall, pelvic bones, anus, and genitalia.4 Several variants of classical bladder exstrophy have been described.6 On this variant, the bladder mucosa is not exposed through the opening of the abdominal wall but has a communicating tract from the bladder to the skin. In 1 series of classic exstrophy only 1 vesical fissure was found among 72 patients.5
Detection of vesical fissure requires examination in a well-lit room with careful attention to any unusual diaper findings. Evaluation of a midline suprapubic dimple found on physical examination requires an ultrasound of the abdominal wall and a renal ultrasound to check for any communicating tract between the anterior abdominal wall and the lower abdominal bladder and to check for any other renal anomalies. Evaluation with a VCUG for the urinary system is recommended. Plain radiograph of the pelvis is done to check for widening of the symphysis pubis. Our patient had a normal renal ultrasound and X-rays of the pelvis. The abdominal ultrasound showed a tract from the lower anterior bladder to the suprapubic lower anterior abdominal wall. A UTI prophylaxis was recommended for potential exposure of the bladder to abdominal wall mucosa. Patient followed up with urology for surgical excision of the tract.
Patient outcome
Unlike patients with bladder exstrophy, patients with vesical fissure have a normal urethra and no problems with urinary incontinence. Because in vesical fissure there is no exposed bladder mucosa, risk of adenocarcinoma of the bladder is absent. This risk of adenocarcinoma of the bladder is present in bladder exstrophy because of bladder mucosa on the anterior abdominal wall; it typically appears between the third and sixth decade of life.7
At 4 months our patient underwent excision of the tract, with a normal exam 2 months later. She continued to track at the 75th percentile for weight and 60th percentile for height and achieved developmental milestone appropriately.
References
1. Kulshrestha S, Kulshrestha M, Yadav A. Complete bladder exstrophy with a normal phallus: a variant of superior vesical fissure. Journal of Pediatric Surgery 2002; 9:1354-1356.
2. TE Herman, MJ Siegel, and PF Austin. Superior vesical fissure; variant classical bladder exstrophy. Journal of Perinatology. 2007; 27:193-195.
3. Siffel C, Correa A, Amar E. Bladder exstrophy: an epidemiologic study from the international clearinghouse for birth defects surveillance and research, and an overview of the literature. Am J Med Genet C Semin Med Genet 2011; 157:321-322.
4. Maruf M, Benz K, Jayman J, Kasprenski M, Michaud J, Di Carlo Heather, Gearhart J. Variant Presentations of the exstrophy-epispadias complex: a 40 year experience. Pediatric Urology. 2019; 125:184-190.
5. DeRiese W, Warmbold H. Adenocarcinoma in exstrophy of the bladder. A Case Report and Review of the Literature: Int Urol and Nephrol 1986; 18:159-162.
6. Bruce S, Duffy JO, Wolf JE Jr. Skin dimpling associated with midtrimester amniocentesis. Pediatr Dermatol. 1984; 2:140-142.
7. Kumar A, Kanojia R, Saili A. Skin dimples. International Journal of Dermatology 2014; 53:789-797.

Artificial intelligence improves congenital heart defect detection on prenatal ultrasounds
January 31st 2025AI-assisted software improves clinicians' detection of congenital heart defects in prenatal ultrasounds, enhancing accuracy, confidence, and speed, according to a study presented at SMFM's Annual Pregnancy Meeting.