What to do in neurologic emergencies
Pediatricians must be able to identify emergent neurologic problems when they present in the office or are suspected in children and proceed with appropriate workup and treatment.
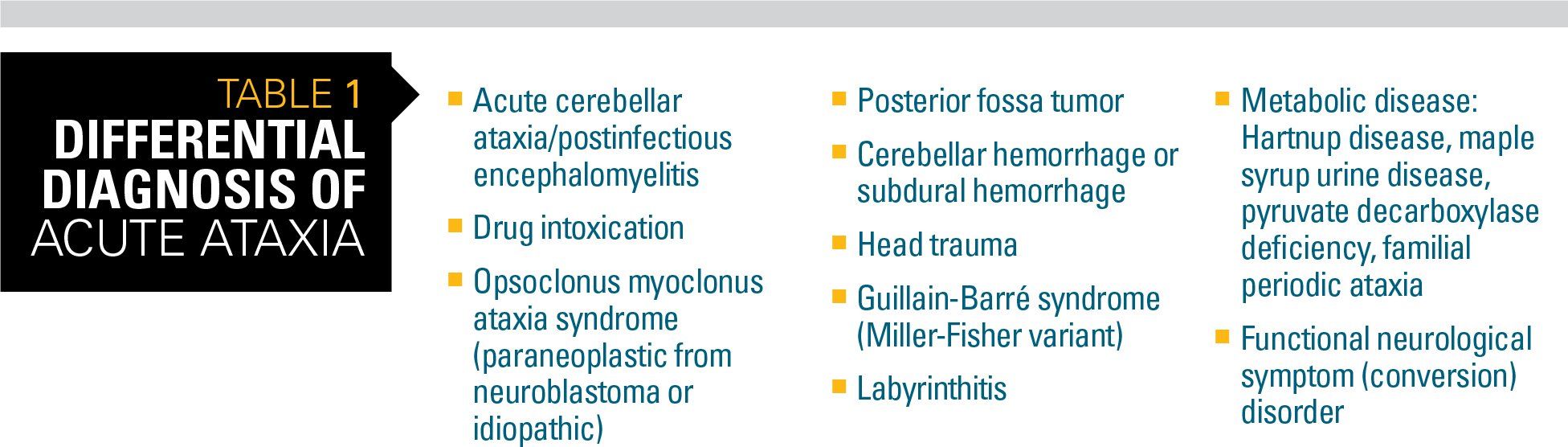
Table 1
ICD-10 codes for diagnosing emergent neurologic symptoms

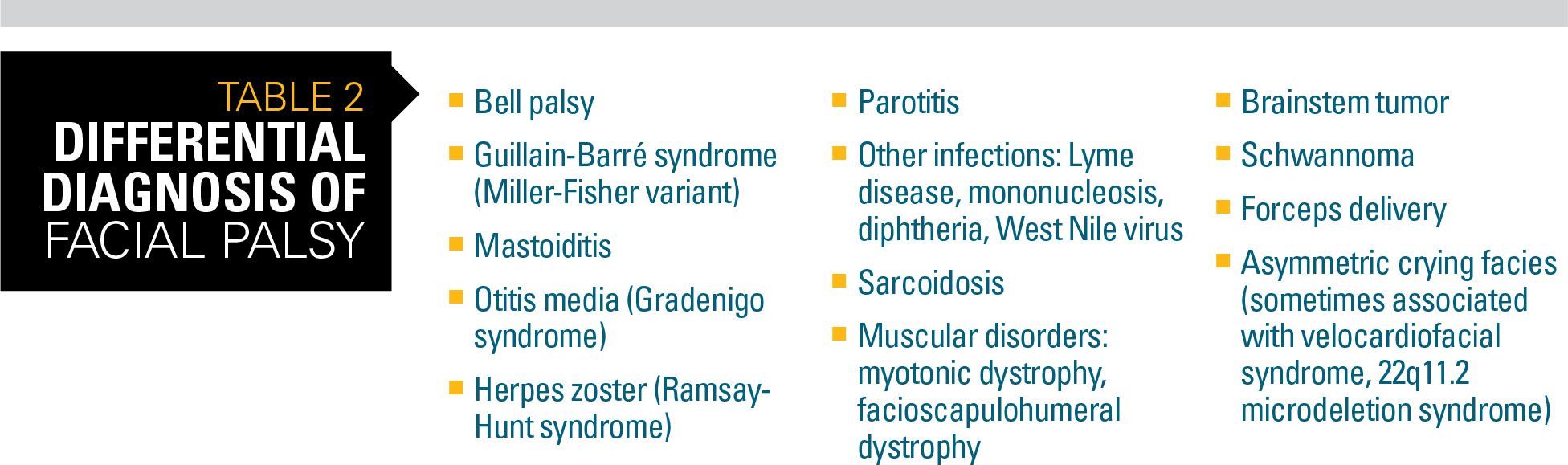
Table 2
Whereas neurologic problems are common in pediatric practice, pediatric neurologic emergencies are relatively uncommon. It is essential for the pediatrician to be able to identify these emergencies, and the pediatrician’s comfort level in addressing these problems is directly related to practice experience, often post residency. However, only about 20% of pediatricians report being “very comfortable” managing childhood neurologic problems, and many practicing pediatricians seek additional education post residency.1 Given workforce issues within pediatric neurology, the pediatrician may not have immediate access to pediatric neurology consultation.2
This paper will review 3 conditions that can present in pediatric practice and identify an appropriate differential diagnosis and workup for each.
CASE 1: 2-year-old with ataxia
A 2-year-old child presents with a several-day history of low-grade fevers, irritability, jerking of extremities, abnormal eye movements, and an unsteady gait. Exam reveals opsoclonus, myoclonus, and impressive ataxia. Head circumference is 46.0 cm (10%).
Acute ataxia is a relatively uncommon presentation to the general pediatrician. Whereas most children have a benign, self-limited problem, the pediatrician must ensure that life-threatening conditions such as a brain tumor or central nervous system (CNS) infection are not present.
Acute ataxia may be defined as an unsteadiness of walking or other fine motor movement that is less than 72 hours old in a previously well child. Most children with acute ataxia present with a refusal to walk or development of a wide-based, drunk-like gait.3
The differential diagnosis for acute ataxia is wide and includes acute infectious processes, postinfectious inflammatory conditions, drug ingestions, and other toxins, tumors, and trauma (Table 1).
However, a recent study looking at 11 years of referrals to a Division of Neurology at a large, urban pediatric medical center revealed cases of acute ataxia were primarily attributed to 3 diagnoses:4
· Postinfectious cerebellar ataxia
· Drug intoxication
· Opsoclonus myoclonus (ataxia) syndrome (OMS/OMAS)
Acute postinfectious cerebellar ataxia is most common in children aged 1 to 6 years and is thought to be triggered by antecedent viral illnesses. Gait ataxia is universal. Twitching of the trunk and head (titubation), action tremor, and end-gaze nystagmus are also common. If alteration of consciousness or multifocal neurologic abnormalities are present, alternative diagnoses such as acute disseminated encephalomyelitis (ADEM) or brainstem encephalitis should be considered.3 The possible alternative diagnosis of opsoclonus myoclonus (ataxia) syndrome (OMS/OMAS) should always be considered.
Ataxia is common after an ingestion of anticonvulsants, benzodiazepines, alcohol, or antihistamines. Accidental poisoning is common in children aged younger than 6 years and in adolescents for whom it is possibly a sign of substance abuse. Alterations of consciousness such as lethargy, slurred speech, or confusion are also common after an accidental ingestion.3
In OMS/OMAS, children will present with extreme irritability, rapid muscle twitches (myoclonus) that worsen with action, and ataxic gait. The characteristic eye movement problem of opsoclonus (ie, rapid, multidirectional saccades) may occur only intermittently, causing the diagnosis to be missed. This syndrome can be idiopathic or paraneoplastic, a presenting sign of neuroblastoma.5
The pediatrician’s main focus is to exclude serious and life-threatening causes of ataxia. A thorough history and physical examination are essential in identifying possible cause of ataxia and in deciding a course of action. Signs and symptoms of recurring or frequent headaches (especially progressive early-morning or evening pattern), double vision, or vomiting suggest possible mass lesion or increased intracranial pressure. Recent head or neck trauma should prompt evaluation for vertebral artery dissection. Cranial nerve abnormalities such as papilledema and cranial nerve palsies suggest an intracranial lesion or hydrocephalus. Whereas nystagmus is seen in both benign and more serious conditions that impact the cerebellar region, pupillary abnormalities are more concerning and can be seen with mass lesions, elevated intracranial pressure, stroke, or intoxication.3
Although physical exam can be difficult in ataxic children because they are already hesitant and irritable, it can provide important clues. As previously stated, children with acute cerebellar ataxia are normally alert and able to interact. Abrupt changes in responsiveness suggest possible ingestions, stroke, or acute disseminated encephalomyelitis. Likewise, asymmetric strength, tone, or reflexes are not common with more benign causes of ataxia in children and should prompt alternative diagnoses and workup.3
Urine and serum drug screens are often diagnostic even when an etiology is not immediately apparent. Other basic lab tests can quickly identify hypoglycemia or suggest a possible inborn error of metabolism if the history is suggestive (eg, amino acids, serum lactate, pyruvate, or ammonia levels). Spinal fluid should be obtained if there is a concern for a CNS infection.
Altered levels of consciousness, focal neurologic signs, cranial nerve abnormalities, concern for a mass lesion, or a history of trauma all are indications for acute neuroimaging. When available, magnetic resonance imaging (MRI) is the imaging modality of choice because of its increased ability to detect posterior fossa lesions such as tumors, strokes, and abscesses.
Treatment depends on the underlying etiology. Most children with postinfectious acute cerebellar ataxia begin to improve quickly and have a complete recovery within several months.
CASE 2: The seizing child
A 3-year-old female on oxcarbazepine (Trileptal) for known epilepsy presents to the emergency department (ED) with a generalized seizure for 20 minutes unresponsive to rectal diazepam (Diastat) 5 mg at home.
In the child with active, generalized seizures, a brief physical exam should assess respiratory and circulatory status while supportive therapies are immediately initiated (eg, oxygen, frequent vitals, airway). Secure intravenous (IV) access should be achieved for blood sampling and administration of medication. Rapid neurologic exam and history from parent or caregiver should be obtained to ascertain possible causes or precipitants of the seizures.
A child is considered to be in status epilepticus (SE) if a single seizure lasts more than 5 minutes or if there are a series of seizures over a 30-minute period without a return to baseline mental status.
Initial workup should include:
· Serum and finger-stick glucose
· Serum electrolytes, calcium, magnesium, and phosphorus levels
· Arterial blood gas and pH
· A complete blood count
· Urine and blood toxicology
· Serum antiseizure drug levels
Subtherapeutic antiseizure drug levels are found in up to 33% of patients presenting with SE.6 Other testing (eg, blood cultures and lumbar puncture for infection; metabolic studies for inborn errors of metabolism) is indicated if history or physical exam are suggestive. Neuroimaging is generally deferred until the patient is stable unless lumbar puncture is being considered wherein computed tomography (CT) is recommended to rule out a mass lesion with risk for herniation prior to the procedure.
A number of different treatment protocols for the treatment of SE are available but there are little comparative data between them. The following briefly outlines treatment supported by the Neurocritical Care Society guideline.7
Initial treatment is with a benzodiazepine (eg, lorazepam 0.1 mg/kg IV up to 4 mg max or diazepam 0.2 mg/kg IV max dose 8 mg) slow pushed over 1 minute. If the child is on a home seizure medication, an additional dose of that medication may be given, if feasible. Supportive care, continuous monitoring, treatment of hypoglycemia, and correction of electrolyte abnormalities should be provided. If seizures continue after 3 to 5 minutes, a second benzodiazepine dose may be given. If 2 or more doses of benzodiazepines are given, the pediatrician should be prepared to perform intubation due to respiratory depression.6-8
If seizures persist after 2 doses of a benzodiazepine, treatment for refractory SE should be started. Although there is no clear evidenced-based treatment recommendation, one commonly recommended treatment option is fosphenytoin at a bolus dose of 20 mg or 30 mg phenytoin equivalents (PE)/kg IV. If seizures still persist after an additional 10 minutes, an additional 5 mg to 10 mg PE/kg IV bolus of fosphenytoin can be administered. Alternatively, IV valproate or levetiracetam could be used.
If seizures persist despite a benzodiazepine and fosphenytoin or other antiseizure medication, a third-line drug such as IV valproate, phenobarbital, levetiracetam, or lacosamide should be initiated. Given the probability for ongoing seizures, the pediatrician should prepare for the possible need for a continuous infusion of midazolam, propofol, or pentobarbital. The child will need the intensive care unit and pressor support is likely to be required.
If IV access is not able to be quickly achieved, alternative routes of administration such as buccal, intranasal, or intramuscular midazolam, rectal diazepam, or intramuscular fosphenytoin should be pursued quickly so as not to delay treatment. However, these routes should not be considered if IV access is able to be obtained, as IV is considered more effective.8
When the seizures stop and the child enters a post-ictal state, it is important to repeat a full neurologic exam to identify any possible causes. If SE is the initial presentation of seizures in a child, neuroimaging is indicated. Likewise, lumbar puncture is indicated if there is any reason to suspect a CNS infection.
CASE 3: Adolescent with drooping face
A 14-year-old boy presents to the office with a left-sided facial droop after a teacher noted his smile was crooked. He admits to abnormal-tasting food. When asked to close his eyes tightly, the left eye is not able to be closed completely and the left side of his mouth appears to droop. No lesions in the ear are noted.
The facial nerve supplies motor innervation to all parts of the face; parasympathetics to the submandibular, sublingual, salivary, and lacrimal glands; and taste fibers for the anterior two-thirds of the tongue. As a result, there are a number of infectious, cancerous, traumatic, idiopathic, or congenital etiologies in the differential (Table 2).
Bell palsy, characterized by rapid onset, unilateral weakness of the upper and lower face, presents with drooping eyelid, loss of nasolabial fold, and drooping of the corner of the mouth. It is the most common disorder impacting the facial nerve (ie, cranial nerve VII). It is responsible for approximately 80% of facial mononeuropathies.9
In addition to the acute (usually over 1 to 2 days) unilateral facial weakness, patients may complain of:9,10
· Decreased forehead movement
· Earache
· Hyperacusis
· Numbness of the face, tongue, and ear
· Taste disturbances
· Tinnitus
Facial movements are assessed by observing the child’s response to specific instructions such as:10
· Closing the eyes
· Elevating the eyebrow
· Smiling and frowning
· Puckering the lips
· Showing the teeth
In children not yet able to follow commands, observing while crying is generally adequate to observe the facial movements.
On examination, Bell phenomenon is a pathognomonic sign (upward movement of the eye on attempting to close the eyelid).9 The child’s ear also should be examined for vesicles or scabbing that would indicate zoster and possible Ramsey-Hunt syndrome.
One key concern is whether the cause is a central (brain or brainstem) versus peripheral (facial nerve) lesion. The strength of forehead muscles is a critical feature. Weakness of eyebrow raising and eye closure supports facial nerve localization. Lower face weakness with sparing of the forehead supports central localization. If accompanied by ipsilateral distal weakness in the arm and hand, a brain problem may be present. Similarly, concurrent inability to abduct the ipsilateral eye suggests a potential brainstem lesion.
Imaging may be necessary if physical signs are atypical but it is not required for all patients with Bell palsy. Similarly, neurodiagnostic testing and lumbar puncture are recommended if history or physical exam point to a disease process where these tests may be diagnostic. Lyme serologies, on the other hand, are recommended in any child with acute onset facial weakness for whom there is a possibility of exposure.9
Treatment is guided by the underlying etiology, with most data coming from studies in adults. In the case of idiopathic facial or Bell palsy, treatment with oral steroids increases the likelihood of full recovery. Benefit from oral acyclovir is possible. Expert opinion seems to recommend antivirals for more severe disease. A 2012 evidence-based guideline from the American Academy of Neurology stated that it was “highly likely” antivirals would not produce a moderate (defined as a >7% difference between those treated and not) improvement in facial function, but that because of the nature of the studies a benefit could not be excluded.11
Conclusion
Whereas pediatric neurologic emergencies are uncommon in pediatric practice, it is essential for the pediatrician to identify when they are present or suspected and proceed with appropriate workup and treatment.
References:
1. Albert DV, Patel AD, Behnam-Terneus M, et al. Child neurology education for pediatric residents. J Child Neurol. 2017;32(3):293-300.
2. Kang PB, Bale JF Jr, Mintz M, et al; Section on Neurology Executive Committee of the American Academy of Pediatrics, and the Board of Directors of the Child Neurology Society. The child neurology clinical workforce in 2015: Report of the AAP/CNS Joint Taskforce. Neurology. 2016;87(13):1384-1392.
3. Ryan MM, Engle EC. Acute ataxia in childhood. J Child Neurol. 2003;18(5):309-316.
4. Thakkar K, Maricich SM, Alper G. Acute ataxia in childhood: 11-year experience at a major pediatric neurology referral center. J Child Neurol. 2016;31(9):1156-1160.
5. Matthay KK, Blaes F, Hero B, et al. Opsoclonus myoclonus syndrome in neuroblastoma: a report from a workshop on the dancing eyes syndrome at the Advances in Neuroblastoma Meeting in Genoa, Italy, 2004. Cancer Lett. 2005;228(1-2):275-282.
6. Riviello JJ, Ashwal S, Hirtz D, et al; American Academy of Neurology Subcommittee; Practice Committee of the Child Neurology Society. Practice parameter: diagnostic assessment of the child with status epilepticus (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2006;67(9):1542-1550.
7. Brophy GM, Bell R, Claassen J, et al; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3-23.
8. Chin RF, Neville BG, Peckham C, Wade A, Bedford H, Scott RC. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study. Lancet Neurol. 2008;7(8):696-703.
9. Zandian A, Osiro S, Hudson R, et al. The neurologist’s dilemma: a comprehensive clinical review of Bell’s palsy, with emphasis on current management trends. Med Sci Monit. 2014;20:83-90.
10. Shargorodsky J, Lin HW, Gopen Q. Facial nerve palsy in the pediatric population. Clin Pediatr (Phila). 2010;49(5):411-417.
11. Gronseth GS, Paduga R; American Academy of Neurology. Evidence-based guide-line update: steroids and antivirals for Bell palsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2012;79(22):2209-2213.
