What you need to know about providing emergency contraception
This update answers key questions about emergency contraception and will help increase your patients' access to this form of pregnancy prevention. Includes a Guide for Patients.
DR. CONARD is assistant professor of pediatrics and DR. GOLD is associate professor of pediatrics at the University of Pittsburgh School of Medicine. They have nothing to disclose in regard to affiliations with, or financial interests in, any organization that may have an interest in any part of this article.
The adolescent pregnancy rate in the United States has been declining since 1991-to a point at which, in 2002, there were 43 births for every 1,000 women 15 to 19 years of age.1 The increasing use of contraceptives by adolescents has been linked to this trend, but adolescents still have a higher rate of contraceptive failure than do older women.2,3
Even when used perfectly, no contraceptive is 100% effective, and adolescents do not always protect themselves adequately-or at all. Timely use of emergency contraception (EC) could reduce the risk of pregnancy by as much as 89% to 95%, depending on the type of oral EC used.4
Once called the morning-after pill or postcoital contraception, emergency contraception is a means of preventing pregnancy after unprotected or underprotected intercourse. The term "morning-after pill" has fallen out of favor because it conveys a limited time frame for use-the morning after intercourse. In fact, EC can be used immediately after and for as long as 120 hours (five days) following intercourse.4
In the 1960s, high-dose oral estrogens were administered for five days as EC, but a high rate of nausea and vomiting limited their use. In 1974, a combination EC method called the Yuzpe regimen was developed that lowered the total estrogen dose and added a progestin.5 This new regimen had fewer side effects than the high-dose estrogen regimen and did not significantly decrease efficacy.6
The first prepackaged formulation of the Yuzpe regimen, called Preven, was approved by the Food and Drug Administration in 1998. In the 1990s, high doses of oral levonorgestrel (a progestin) were found to be effective for EC.7-9 Plan B, a levonorgestrel-only product, was approved by the FDA in 1999. The Preven brand was purchased by the makers of Plan B, Barr Laboratories, but taken off the market in 2004 because of the superior efficacy and side effect profile of Plan B.
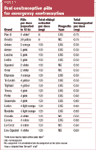
What products can be used for EC?
Two types of POPs are available in the US. Plan B is a dedicated product that contains two tablets (0.75 mg each) of levonorgestrel. Ovrette is a progestin-only OC usually used as ongoing contraception for women with medical contraindications to estrogen. When used for EC, 20 tablets of Ovrette per dose taken 12 hours apart (for a total of 40 tablets) is required to obtain the necessary dose of levonorgestrel.
The FDA-approved instructions for Plan B are to take one tablet as soon as possible after unprotected intercourse and to take the second tablet 12 hours later. However, recent data show that taking the two Plan B tablets at the same time is as effective at preventing pregnancy and does not cause more side effects.10 The FDA instructions also state that the first Plan B dose can be started as late as 72 hours after unprotected intercourse, but recent data support starting the regimen as late as 120 hours after coitus.11,12
Plan B is generally priced at $25 to $35 for a packet (for the two-tablet regimen) but may be more expensive in some pharmacies-or unavailable. Ovrette can cost as much as $70 for the two packs needed to complete the full progestin-only regimen. In states where pharmacists can dispense EC, cost may be $50 to $55, which includes the $10 counseling fee charged by the pharmacist.12
Having "the talk" with teen patients
June 17th 2022A visit with a pediatric clinician is an ideal time to ensure that a teenager knows the correct information, has the opportunity to make certain contraceptive choices, and instill the knowledge that the pediatric office is a safe place to come for help.
Meet the Board: Vivian P. Hernandez-Trujillo, MD, FAAP, FAAAAI, FACAAI
May 20th 2022Contemporary Pediatrics sat down with one of our newest editorial advisory board members: Vivian P. Hernandez-Trujillo, MD, FAAP, FAAAAI, FACAAI to discuss what led to her career in medicine and what she thinks the future holds for pediatrics.