CAR T-cell therapy in pediatrics
Recent developments in chimeric antigen receptor (CAR) T-cell therapy have shown promise in treating relapsed B-cell acute lymphoblastic leukemia in children.
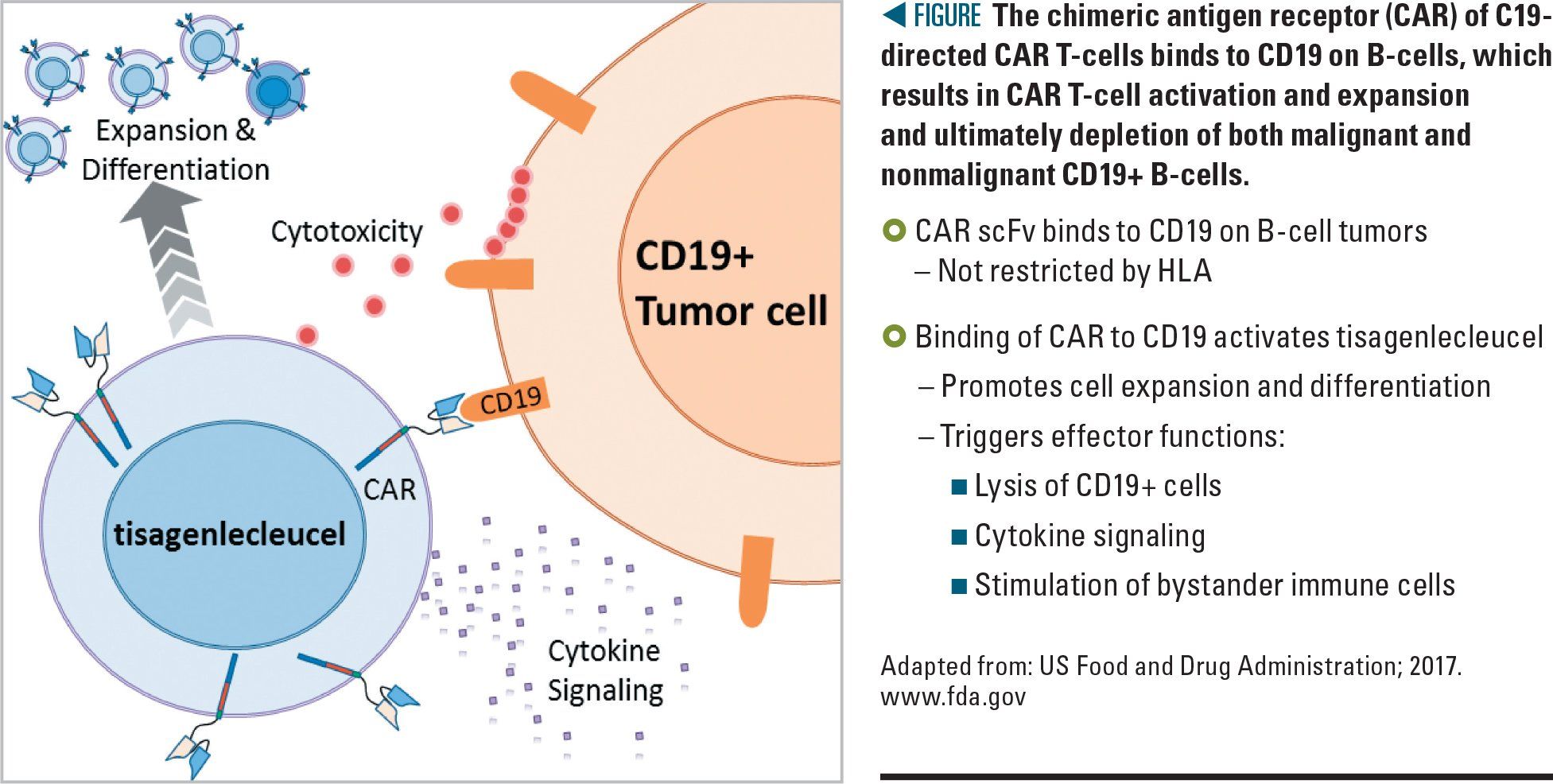
Figure
Note from Dr Lee

Leukemia is the most common malignancy in children, representing nearly 30% of pediatric cancers. B-cell acute lymphoblastic leukemia (B-ALL), which represents nearly 80% of ALL, is a disease in which most pediatric patients are cured using standard chemotherapy. However, there is a subset of these patients, approximately 20%, for whom standard chemotherapy is ineffective. Once relapsed, the survival rates have historically been poor, with long-term survival less than 20% in patients with early relapses, and 50% in those with relapses greater than 36 months from diagnosis.
Due to the high prevalence of ALL relative to other pediatric malignancies, this relapsed population is substantial. Chimeric antigen receptor T-cells (CAR-T) targeting CD19+ B-cells is an innovative new therapy that has achieved 80% to 90% complete remission rates in patients with relapsed or refractory B-ALL, with some products achieving durable remissions greater than 12 months in approximately half of pediatric patients.1
What are CAR T-cells?
CAR T-cells redirect a patient’s immune system toward cancer cells and, in its idealized conceptualization, the treatment is the equivalent of administering a living persisting drug. Chimeric antigen receptors are synthetically engineered receptors against a selected tumor antigen, and are incorporated into T-cells to redirect and enhance their function against the cancer cell.2
Although a CAR T-cell can be engineered to target any receptor, those targeting CD19 on B-cell malignancies have been the best-studied therapies to date, and there are now 2 CD19 CAR T-cell products available on the market (Figure). The first, Kymriah (tisagenlecleucel) by Novartis Pharmaceuticals (East Hanover, New Jersey), was approved by the US Food and Drug Administration (FDA) in August 2017.3 The second CAR T-cell product, Yescarta (axicabtagene ciloleucel) by Kite Pharma (Foster City, California), was FDA approved in October 2017.4 Using CAR T-cells to treat other tumor types has thus far been limited by the identification of similarly effective target antigens with limited on-target adverse effects, as well as an immunosuppressive environment that protects many solid tumors from infiltrating T-cells.5
The process of manufacturing CAR T-cells begins by collecting the patient’s T-cells via apheresis, which are then genetically engineered to express the CAR gene using an inactivated viral vector. These cells are then expanded in the laboratory and undergo quality control assessments. During this time, the patient undergoes a short conditioning chemotherapy regimen to deplete remaining lymphocytes. The newly engineered T-cells are then infused into the patient, with a “vein-to-vein” time of several weeks.6
Adverse effects
Like any cancer treatment, CAR T-cell therapy is not without unique adverse effects.7 However, unlike toxic effects associated with chemotherapies or bone marrow transplants, CAR T-cell adverse effects are reversible in nearly all cases. As these engineered T-cells become activated and release proinflammatory cytokines, up to 80% of patients experience cytokine release syndrome (CRS).1,3 This cardinal toxicity typically develops within 1 to 3 weeks after administration and is similar in presentation to sepsis, including fever, hypotension, tachycardia, and capillary leak, which can progress to hypoxia, respiratory failure, and/or shock.7,8 Approximately, 50% of patients will require a brief intensive care admission for blood pressure and respiratory support.1
Treatment of CRS involves supportive care and reduction of cytokines with tocilizumab, an IL-6 antagonist, originally used for rheumatoid arthritis. Because of the lifesaving potential of tocilizumab for CRS, centers administering CAR T-cells are required by the FDA to have 2 doses of tocilizumab on hand for each patient.3 For CRS unresponsive to supportive care measures and tocilizumab, corticosteroids may be considered. However, due to their immunosuppressive properties, there is some concern that corticosteroids may impact the efficacy of CAR T-cells. An oncologist should always be involved when considering steroid use in patients who have recently received CAR T-cells. The presence of CRS is most prominent in patients with high disease burden and reflects the fact that active CAR T-cells are performing their cytotoxic function. In fact, patients who do not develop CRS are less likely to see a response to therapy.2
Additionally, 30% to 45% of patients will experience a spectrum of neurotoxicity known as CAR T-cell–related encephalopathy syndrome (CRES)1,7,8 that may include delirium, encephalopathy, aphasia, confusion, seizure, hallucination, and, rarely, cerebral edema. The mechanism of these neurotoxic symptoms is not yet known. Delirium screening and neurologic assessment are performed at least daily until 4 weeks after the CAR T-cell infusion. Additionally, antiseizure prophylaxis is recommended during this high-risk period, especially for patients with a history of central nervous system disease or seizures. Tocilizumab is not effective for CRES and, therefore, management is typically limited to supportive care. In severe cases, steroids may be considered but, as with their use in CRS, they may jeopardize the efficacy of the CAR T-cells and thus should never be given without the involvement of an oncologist.8
Most long-term effects of CAR T-cells remain unknown given the novelty of this therapy. However, by targeting CD19, patients who receive CD19 CAR T-cells will develop aplasia of their normal B-cells, and consequently the inability to produce antibodies.1 This is not only an expected outcome, but one that renders CD19 a desirable and well-studied target because this aplasia can be easily managed with regular immunoglobulin replacement. These patients also require deferral of vaccinations for as long as the aplasia persists.8
Next steps
The future for CAR T-cell therapy is bright. There have emerged more than 100 CAR T-cell companies investigating nearly 50 tumor biomarker targets. Patients who relapse after CD19 CAR T-cells may do so because their leukemia or lymphoma cells no longer express CD19, a phenomenon called antigen loss. Thus, future targets for these disease states include other leukemia receptors, such as CD22 and CD20. Additionally, CAR T-cell therapy is being explored for other solid tumor malignancies including neuroblastoma and melanoma.9
Ongoing challenges involving CAR T-cells are numerous and include: feasibility across more institutions; management of relapse after CAR T-cell therapy; reversing endogenous T-cell exhaustion; and expanding the selection of patients who are most likely to derive benefit from these novel agents.8
Finally, the scope of CAR T-cell therapy is not limited to oncologic care but also has the potential to extend to autoimmune, infection, and degenerative conditions.10
