A pediatrician’s dilemma: Understanding, diagnosing, and treating antenatal hydronephrosis
Antenatal hydronephrosis (ANH), also referred to as urinary tract dilation, is one of the most confounding challenges to the practicing pediatrician. When dealing with newborns and children with ANH, pediatricians face understanding the significance of the degree of hydronephrosis, risk assessment, prenatal and postnatal management, and the use of prophylactic antibiotics.
A pragmatic and clinically focused approach to these issues should facilitate the pediatrician in their treatment of infants with prenatally detected hydronephrosis.
The use of routine fetal ultrasound has increased the diagnosis of ANH. ANH, which is dilation of the fetal renal collecting system, is one of the most common fetal anomalies, occurring in 1% to 5% of pregnancies.1,2 However, Hamilton et al reported an incidence of 1% to 2% of pregnancies in the United States, or 40,000 to 80,000 cases annually based on an estimated annual birth rate of 4 million.3 Prenatal ultrasound, which can visualize the kidneys at 12 to 13 weeks’ gestation and identify distinct renal architecture by week 20, has the advantage of allowing follow-up without invasive testing or radiation exposure. Fetal magnetic resonance imaging may be indicated in select cases to further delineate the structural abnormalities of the fetal urinary tract.
ANH may be a sign of underlying severe fetal urinary system anomalies or chromosomal aneuploidies and also may be associated with impaired renal development and fetal renal parenchymal injury.2,4 ANH may be transient in 50% to 70% of cases. It also may be secondary to ureteropelvic junction (UPJ) obstruction in 10% to 30% of cases, vesicoureteral reflux (VUR) in 10% to 40% of cases, ureterovesical junction (UVJ) obstruction in 5% to 15% of cases, multicystic dysplastic kidney in 2% to 5% of cases, posterior urethral valves (PUV) in 1% to 5% of cases, and other anomalies.5 Due to the increase in the detection of ANH, pediatricians had increased responsibility in administering parental prenatal counseling and pre- and postnatal diagnostic and treatment plans. The rationale for early diagnosis of ANH is to treat urinary tract obstruction and prevent pyelonephritis, renal calculi, loss of renal function, and chronic kidney disease.
Table 1
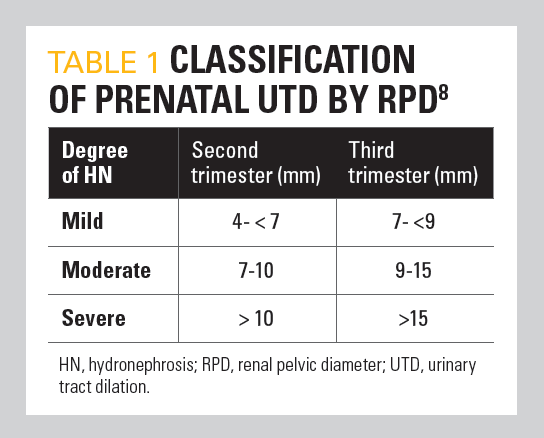
Degree of hydronephrosis
Three systems are used to classify the degree of ANH: renal pelvic diameter (RPD), Society for Fetal Urology (SFU) criteria, and urinary tract dilation (UTD) classification. Although not uniformly accepted, the most common method to determine RPD is measurement of the maximum anteroposterior diameter of the renal pelvis in a transverse plane.6 RPD of less than 4 mm at less than 28 weeks’ gestation and less than 7 mm at 28 weeks’ gestation is usually transient and does not usually require postnatal workup or future intervention.7 These authors found that an anteroposterior diameter (APD) of 4 mm or greater before 33 weeks’ gestation or 7 mm or greater after 33 weeks’ gestation was 100% accurate in identifying patients with abnormal renal function or those who might require intervention postnatally. However, many of these patients will demonstrate spontaneous improvement without surgical intervention. Others have classified RPD as mild, moderate, or severe according to specific measurement criteria (Table 18). An RPD of greater than 10 mm in the second trimester is associated with increased risk for the presence of congenital anomalies of the kidney and urinary tract (CAKUT). However, a clear cutoff value for defining obstruction requiring surgical intervention has not been determined.
Table 2
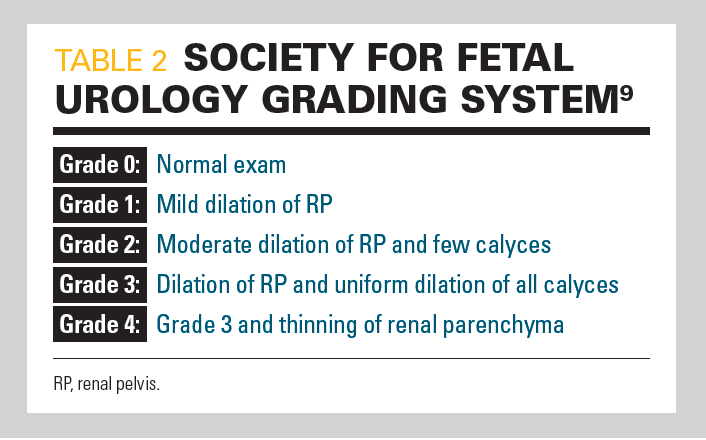
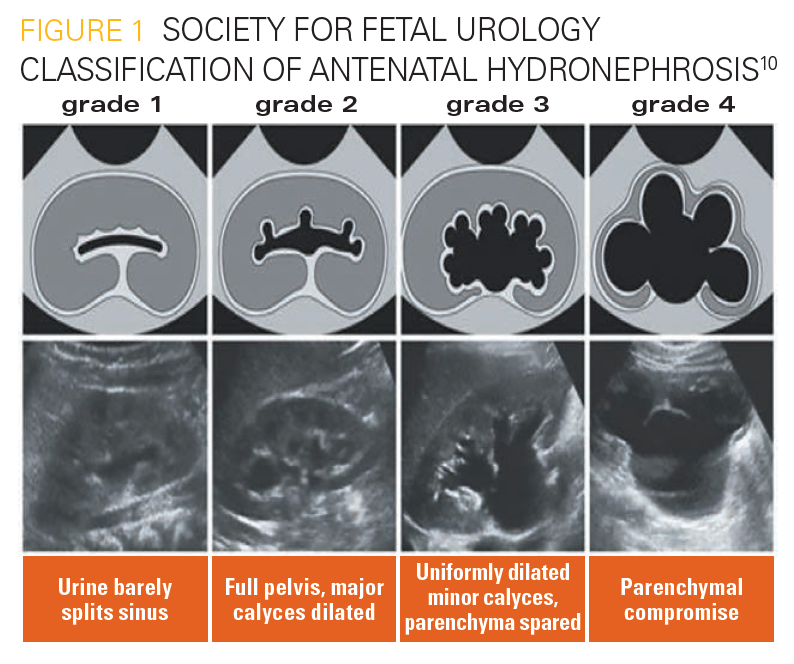
In 1993, the SFU proposed a classification based on the appearance of the calices, renal pelvis, and renal parenchyma to determine the grade of ANH (Table 29) (Figure 110). The SFU grading system is probably the most commonly used system of classification for both pre- and postnatal grading of UTD.5
Figure 1
In 2014, a multidisciplinary work group proposed a revision to the SFU classification, using “UT dilation” as a common terminology that would lead to a more standardized reporting of abnormal findings. This classification requires more extensive modifiers to determine the level of urinary tract obstruction than the SFU system. In the normal fetus, calyceal dilation is absent, the renal parenchyma has normal thickness and appearance, the ureter is not seen, the bladder is normal, and there is no unexplained oligohydramnios (Table 35). According to the new UTD system, the renal pelvis is considered to be not dilated if the APD measures less than 4 mm at less than 28 weeks’ gestation, less than 7 mm at 28 weeks’ gestation or greater, and less than 10 mm postnatally. A fetus is considered to be at increased risk of postnatal uropathy if the APD of the renal pelvis is 7 mm or greater at less than 28 weeks’ gestation, or 10 mm or greater at 28 weeks’ gestation or greater, or if there is 1 of the following: dilation of peripheral calyces, abnormal parenchymal thickness, appearance of visibly dilated ureter, abnormal bladder, or the presence of oligohydramnios.5
Table 3
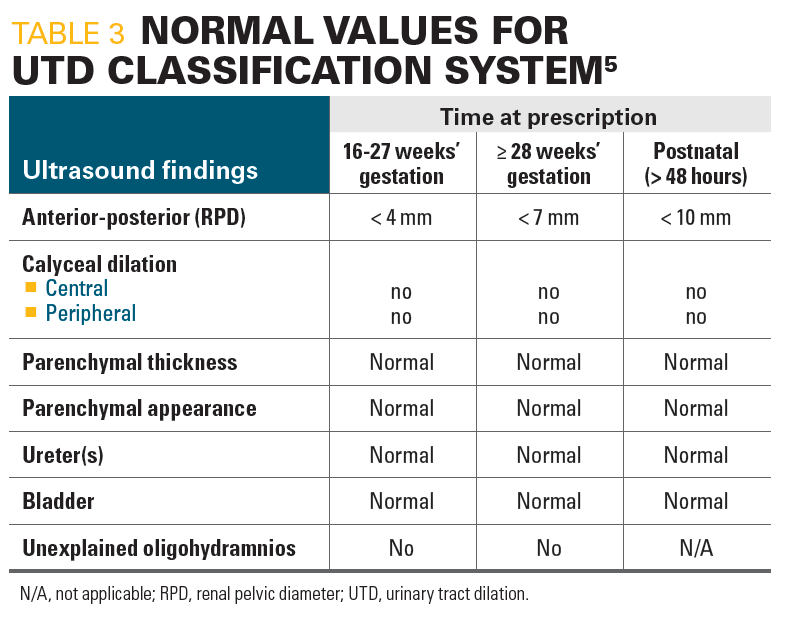
Patients with pathological ANH have high SFU and APD grades, and both grading systems may be used as relatively reliable predictors for the outcome of ANH, including ANH regression and postpartum surgery.11 These authors found that the sensitivity and specificity of the SFU and that of the APD grading system are very similar, indicating that both grading systems have a relatively high diagnostic accuracy. However, they noted that the combination of both grading systems seems to have a higher diagnostic value compared with that of each system alone regarding the discrimination of pathological ANH from physiological ANH.
Prenatal management
Management of ANH depends on the risk assessment. A fetus categorized as low risk based on ultrasound prior to 32 weeks’ gestation will require only 1 further repeat ultrasound at starting at 32 weeks’ gestation. However, ANH (APD > 4 mm in the second trimester, > 7 mm in the third trimester) requires further follow-up. Findings suspicious of PUV (oligohydramnios, dilated bladder, or bilateral ANH) warrant monitoring every 2 to 4 weeks throughout pregnancy, and any comorbid fetal abnormality also should be investigated.12 If increasing oligohydramnios is seen after 20 weeks’ and before 32 weeks’ gestation, bladder outlet obstruction is suspected and fetal intervention such as vesicoamniotic (VA) shunting may be considered. This will allow for the return of amniotic fluid in an effort to promote fetal lung development. The middle of the second trimester is the ideal time period to offer prenatal intervention.
Fetal intervention of VA shunting may improve postnatal outcome in fetuses with lower urinary tract obstruction who have favorable fetal renal function parameters. Fetal renal function can be evaluated by combining fetal urinary biochemistry profiles with ultrasound characteristics of fetal kidneys according to gestational age.13 Increased levels of sodium, calcium, chloride, β2 microglobulin, and decreased levels of creatinine after 18 weeks’ gestation measured in fetal urine in patients with bilateral obstructive uropathy and oligohydramnios are associated with poor neonatal outcomes and may serve as adjuvants to decide on the need for antenatal intervention.14 Morris et al15 reported higher survival in the fetuses receiving VA shunting, whereas Spiro et al16 reported that prenatal interventions do not improve renal function prognosis of patients with oligohydramnios associated with CAKUT.
Postnatal management
Because there is no consensus on the postnatal approach to ANH, fetuses with persistent antenatal renal pelvis measurements 7 mm or greater in the third trimester should be investigated postnatally. Because physiologic dehydration of the newborn can lead to underestimation of hydronephrosis, an early normal postnatal ultrasound, if done in the first 72 hours of life, does not preclude the presence of urinary tract abnormality.17 Lee et al18 screened 1645 citations, of which 17 studies met inclusion criteria and created a data set of 1308 participants. There was a significant increase in risk of any postnatal pathology per increasing degree of ANH (11.9% for mild, 45.1% for moderate, and 88.3% for severe). The risk of VUR was similar for all degrees of antenatal hydronephrosis.
Most cases of ANH are transient and resolve spontaneously with increasing gestational age or soon after birth. Maayan-Metzger et al found that 97.5% of mild prenatal ANH resolved or remained mild on follow-up, although 80% of the moderate and only 20% of the severe cases improved.19 Barbosa et al showed that 25% of ANH resolved prenatally, including 90%, 75%, and 28% of mild, moderate, and severe ANH cases, respectively.20
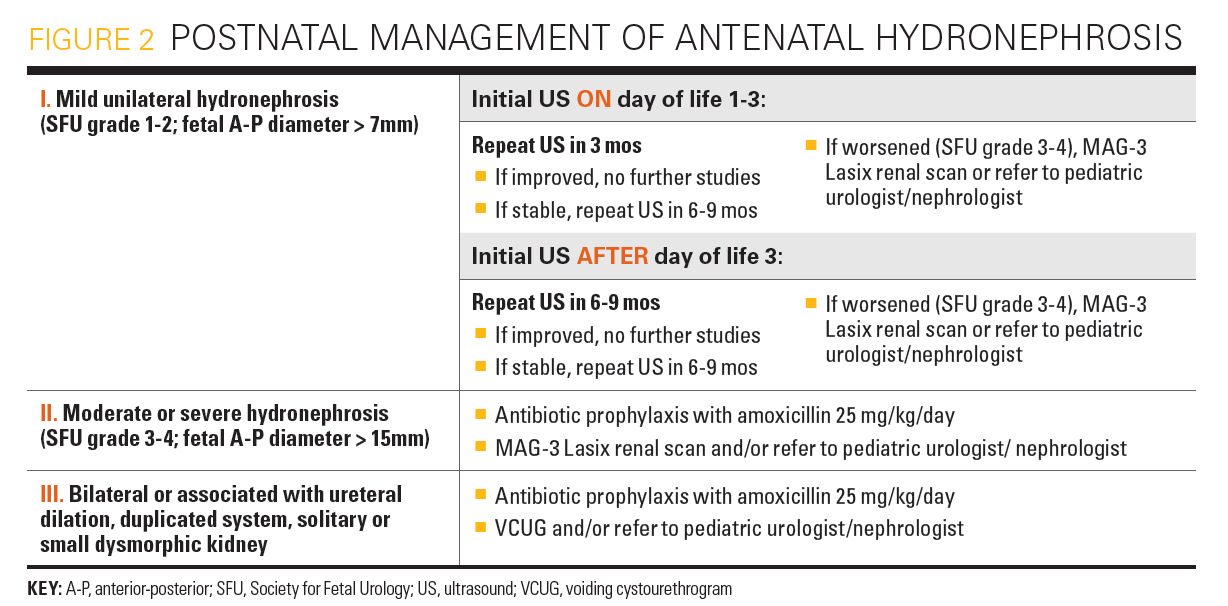
Because hydronephrosis could be underestimated on an early postnatal ultrasound, newborns with persistent or worsening ANH should be evaluated with a postnatal ultrasound after at least 48 to 72 hours of life. Ultrasound findings (SFU grade 1-2) after 48 to 72 hours of life in patients associated with low risk for postnatal uropathy should be followed up with an ultrasound in 3 to 6 months. If the hydronephrosis improves, longer-term imaging is not necessary, based on the very low risk of progression. Patients with higher-risk (SFU grades 3-4) hydronephrosis should undergo more frequent ultrasounds and, when indicated, a MAG-3 Lasix renal scan to rule out obstruction at the UPJ or UVJ. This allows for the evaluation of both differential renal function and drainage of the dilated collecting system. Consultation with a pediatric urologist or nephrologist should also be considered in these higher-risk patients.
Opinions differ on the indication for VCUG in patients with ANH. Many centers screen all patients with grades 3 to 4 hydronephrosis to look for clinically significant dilating VUR (grade > 3). However, more specific selection criteria for VCUG have been proposed, including ureteral dilation, duplication of the collecting system, and/or renal dysmorphia, which were shown to have the same yield for detection of dilating VUR but with only approximately half as many patients requiring VCUG.21
Patients with risk of more severe postnatal uropathy, such as those with suspected PUV, bilateral severe hydronephrosis, or solitary hydronephrotic kidney, should be routinely evaluated with a VCUG and MAG-3 Lasix renal scan during the first 1 to 2 weeks of life to determine renal function, renal drainage, and the possible need for early neonatal surgical intervention versus close serial monitoring with renal function studies and ultrasounds.5,8,22 Prophylactic antibiotics may be considered at this stage. Unfortunately, there are not enough data to support strong recommendations in many instances, and some decisions are left to the discretion of the specialist. An algorithm of the postnatal management of ANH is shown in Figure 2.
Figure 2
Urinary tract infection and the use of prophylactic antibiotics
Female gender, uncircumcised males, high-grade UTD, and VUR were found to be independent high-risk predictors of febrile urinary tract infection (UTI) in patients with ANH.23,24 The benefits of continuous antibiotic prophylaxis in patients with ANH are controversial.25 In a systematic review of the literature, which included 3876 infants, UTI rates in patients with low-grade hydronephrosis were similar regardless of continuous antibiotic prophylaxis status: 2.2% on prophylaxis versus 2.8% not receiving prophylaxis.26,27 However, when looking at high-grade UTD, a significant decrease in the rate of UTI was seen (14.6% treated vs 28.9% untreated; P < .01). The estimated number of patients needed to treat to prevent 1 UTI in those with high-grade UTD was 7.
In a meta-analysis of prenatal UTD in 2008, Lee et al reported a 4%, 14%, 33%, and 40% incidence of UTI in SFU grades of 1 to 4, respectively, which indicated the presence of a linear relationship between the degree of UTD and risk of developing UTI.28 Higher rates of UTI in higher-grade UTD as compared with lower-grade UTD also were reported.27 It appears from these studies that the presence of moderate to severe UTD does place the neonate at risk of UTI. Prophylactic antibiotics may have to be considered to prevent UTI and risk for kidney damage.
Ureteral dilation has shown to be an independent risk factor for UTI.23,24 Herz et al in a 10-year retrospective experience in patients with prenatally detected UTD, reported the incidence of febrile UTI on prophylactic antibiotics to be 7.9% versus 18.7% without treatment.29 The authors found that ureteral dilation of more than 11 mm in patients not maintained on prophylactic antibiotics afforded a 5-fold higher risk of developing a febrile UTI. They also suggested that continuous antibiotic prophylaxis may play a significant role in reducing the risk of febrile UTI in children with ANH and risk factors (ureteral dilation, high-grade VUR, and UVJ obstruction), but otherwise seems unnecessary. Other reports showed that antibiotic prophylaxis did not reduce the risk of UTI, even in the high-risk groups; however, the authors did not specifically investigate the risk in cases associated with ureteral dilation.24 Additionally, in an observational registry from 4 centers, Zee et al were unable to demonstrate an association between UTI and the use of prophylactic antibiotics, presence of VUR, dilated ureter, or renal duplication.30 Only 26% of their 213 patients had SFU grade 3 to 4 hydronephrosis and only 11% had ureteral dilation, 88% of whom were treated with antibiotic prophylaxis. Although the benefit of continuous antibiotic prophylaxis in infants with ANH is not proven, clinicians may decide to use them in patients with ANH associated with other high-risk predictors. Physicians should discuss the rationale of prophylactic antibiotics with the family prenatally and postnatally.
Newborn circumcision is associated with a significantly lower rate of UTI among infant boys with hydronephrosis and should be considered, particularly in newborns with higher risk of infection, including ureteral dilation, PUV, or dilating VUR.24,30 The evaluation/management of antenatal hydronephrosis needs to be tailored based on risk assessment. The use of routine continuous antibiotic prophylaxis in infants with ANH is controversial; however, it may have to be considered in cases associated with ureteral dilatation or dilating (Grade >3) VUR. Newborn circumcision is also an effective measure to reduce the risk of UTI in males.
References
1. Nguyen HT, Herndon CDA, Cooper C, et al. The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J Pediatr Urol. 2010;6(3):212-231. doi:10.1016/j.jpurol.2010.02.205
2. Liu DB, Armstrong WR, Maizels M. Hydronephrosis: prenatal and postnatal evaluation and management. Clin Perinatol. 2014;41(3):661-678. doi:10.1016/j.clp.2014.05.013
3. Hamilton BE, Martin JA, Ventura SJ.Births: preliminary data for 2012.Natl Vital Stat Rep. 2013;62(3):1-20.
4. Mallik M, Watson AR. Antenatally detected urinary tract abnormalities: more detection but less action. Pediatr Nephrol. 2008;23(6):897-904. doi:10.1007/s00467-008-0746-9
5. Nguyen HT, Benson CB, Bromley B, et al. Multidisciplinary consensus on the classification of prenatal and postnatal urinary tract dilation (UTD classification system). J Pediatr Urol. 2014;10(6):982-998. doi:10.1016/j.jpurol.2014.10.002
6. Woodward M, Frank D. Postnatal management of antenatal hydronephrosis. BJU Int. 2002;89(2):149-156. doi:10.1046/j.1464-4096.2001.woodward.2578.x
7. Corteville JE, Gray DL, Crane JP. Congenital hydronephrosis: correlation of fetal ultra-sonographic findings with infant outcome. Am J Obstet Gynecol. 1991;165(2):384-388. doi:10.1016/0002-9378(91)90099-d
8. Ahmad AE, Kogan BA. Congenital hydronephrosis. In: Barakat AJ, Rushton HG, eds. Congenital Anomalies of the Kidney and Urinary Tract: Clinical Implications in Children. Springer International Publishing; 2016:77-93.
9. Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Ped Radiol. 1993;23(6):478-480. doi:10.1007/BF02012459
10. Timberlake MD, Herndon CDA. Mild to moderate postnatal hydronephrosis-grading systems and management. Nat Rev Urol. 2013;10(11):649-656. doi:10.1038/nrurol.2013.172
11. Zhang D, Sun X, Chen X, et al. Ultrasound evaluation for prediction of outcomes and surgical decision in fetal hydronephrosis. Exp Ther Med. 2019;18(2):1399-1406. doi:10.3892/etm.2019.7683
12. Hong YK, Lee JH. Evaluation and management of antenatal hydronephrosis. Child Kidney Dis. 2015;19:8-13. doi:10.3339/chikd.2015.19.1.8
13. Ruano R, Sananes N, Wilson C, et al. Fetal lower urinary tract obstruction: proposal for standardized multidisciplinary prenatal management based on disease severity. Ultrasound Obstet Gynecol. 2016;48(4):476-482. doi:10.1002/uog.15844
14. Lipitz S, Ryan G, Samuell C, et al. Fetal urine analysis for the assessment of renal function in obstructive uropathy. Am J Obstet Gynecol. 1993;168(1):174‐179. doi:10.1016/s0002-9378(12)90909-6
15. Morris RK, Malin GL, Quinlan-Jones E, et al. Percutaneous vesicoamniotic shunting versus conservative management for fetal lower urinary tract obstruction (PLUTO): a randomised trial. Lancet. 2013;382(9903):1496-1506. doi:10.1016/S0140-6736(13)60992-7
16. Spiro JE, Konrad M, Rieger-Fackeldey E, et al. Renal oligo-and anhydramnios: cause, course and outcome-a single-center study. Arch Gynecol Obstet. 2015;292(2):327-336. doi:10.1007/s00404-015-3648-7
17. Aksu N, Yavaşcan O, Kangin M, et al. Postnatal management of infants with antenatally detected hydronephrosis. Pediatric Nephrol. 2005;20(9):1253-1259. doi:10.1007/s00467-005-1989-3
18. Lee RS, Cendron M, Kinnamon DD, Nguyen HT. Antenatal hydronephrosis as a predictor of postnatal outcome: a meta-analysis. Pediatrics. 2006;118(2):586-593. doi:10.1542/peds.2006-0120
19. Maayan-Metzger A, Lotan D, Jacobson JM, et al. The yield of early postnatal ultrasound scan in neonates with documented antenatal hydronephrosis. Am J Perinatol. 2011;28(8):613-618. doi:10.1055/s-0031-1276735
20. Barbosa JABA, Chow JS, Benson CB, et al. Postnatal longitudinal evaluation of children diagnosed with prenatal hydronephrosis: insights in natural history and referral pattern. Prenat Diagn. 2012;32(13):1242-1249. doi:10.1002/pd.3989
21. Lee NG, Rushton HG, Peters CA, Groves DS, Pohl HG. Evaluation of prenatal hydronephrosis: novel criteria for predicting vesicoureteral reflux on ultrasonography. J Urol. 2014;192(3):914-918. doi:10.1016/j.juro.2014.03.100
22. Clayton DB, Brock JW. Lower urinary tract obstruction in the fetus and neonate. Clin Perinatol. 2014;41(3):643‐659. doi:10.1016/j.clp.2014.05.012
23. Braga LH, Farrokhyar F, D’Cruz J, Pemberton J, Lorenzo AJ. Risk factors for febrile urinary tract infection in children with prenatal hydronephrosis: a prospective study. J Urol. 2015;193(suppl 5):1766-1771. doi:10.1016/j.juro.2014.10.091
24. Zareba P, Lorenzo AJ, Braga LH. Risk factors for febrile urinary tract infection in
infants with prenatal hydronephrosis: comprehensive single center analysis. J Urol.
2014;191(suppl 5):1614-1618. doi:10.1016/j.juro.2013.10.035
25. Silay MS, Undre S, Nambiar AK, et al. Role of antibiotic prophylaxis in antenatal hydronephrosis: a systematic review from the European Association of Urology/European Society for Paediatric Urology Guidelines Panel. J Pediatr Urol. 2017;13(3):306-315. doi:10.1016/j.jpurol.2017.02.023
26. Braga LH, Mijovic H, Farrokhyar F, Pemeberton J, DeMaria J, Lorenzo Aj: Antibiotic prophylaxis for urinary tract infections in antenatal hydronephrosis. Pediatrics. 2013;131(1):e251-261. doi:10.1542/peds.2012-1870
27. Szymanski KM, Al-Said AN, Pippi Salle JL, Capolicchio JP. Do infants with mild prenatal hydronephrosis benefit from screening for vesicoureteral reflux? J Urol. 2012;188(2):576-581. doi:10.1016/j.juro.2012.04.017
28. Lee JH, Choi HS, Kim JK, Won H-S, et al. Nonrefluxing neonatal hydronephrosis and the risk of urinary tract infection. J Urol. 2008; 179 (4): 1524-1528. doi.org/10.1016/j.juro.2007.11.090
29. Herz D, Merguerian P, McQuiston L. Continuous antibiotic prophylaxis reduces the risk of febrile UTI in children with asymptomatic antenatal hydronephrosis with either ureteral dilation, high-grade vesicoureteral reflux, or ureterovesical junction obstruction. J Pediatr Urol. 2014;10(4):650-654. doi:10.1016/j.jpurol.2014.06.009
30. Zee RS, Herbst KW, Kim C, et al. Urinary tract infections in children with prenatal hydronephrosis: a risk assessment from the Society for Fetal Urology Hydronephrosis Registry. J Pediatr Urol. 2016;12(4):261.e1-7. doi:10.1016/j.jpurol.2016.04.024

Having "the talk" with teen patients
June 17th 2022A visit with a pediatric clinician is an ideal time to ensure that a teenager knows the correct information, has the opportunity to make certain contraceptive choices, and instill the knowledge that the pediatric office is a safe place to come for help.
Meet the Board: Vivian P. Hernandez-Trujillo, MD, FAAP, FAAAAI, FACAAI
May 20th 2022Contemporary Pediatrics sat down with one of our newest editorial advisory board members: Vivian P. Hernandez-Trujillo, MD, FAAP, FAAAAI, FACAAI to discuss what led to her career in medicine and what she thinks the future holds for pediatrics.