How to diagnose and treat severe asthma
Severe or refractory asthma places a small subset of children with asthma at risk for significant morbidity and treatment challenges, as well as for higher healthcare utilization and costs.
Table 1

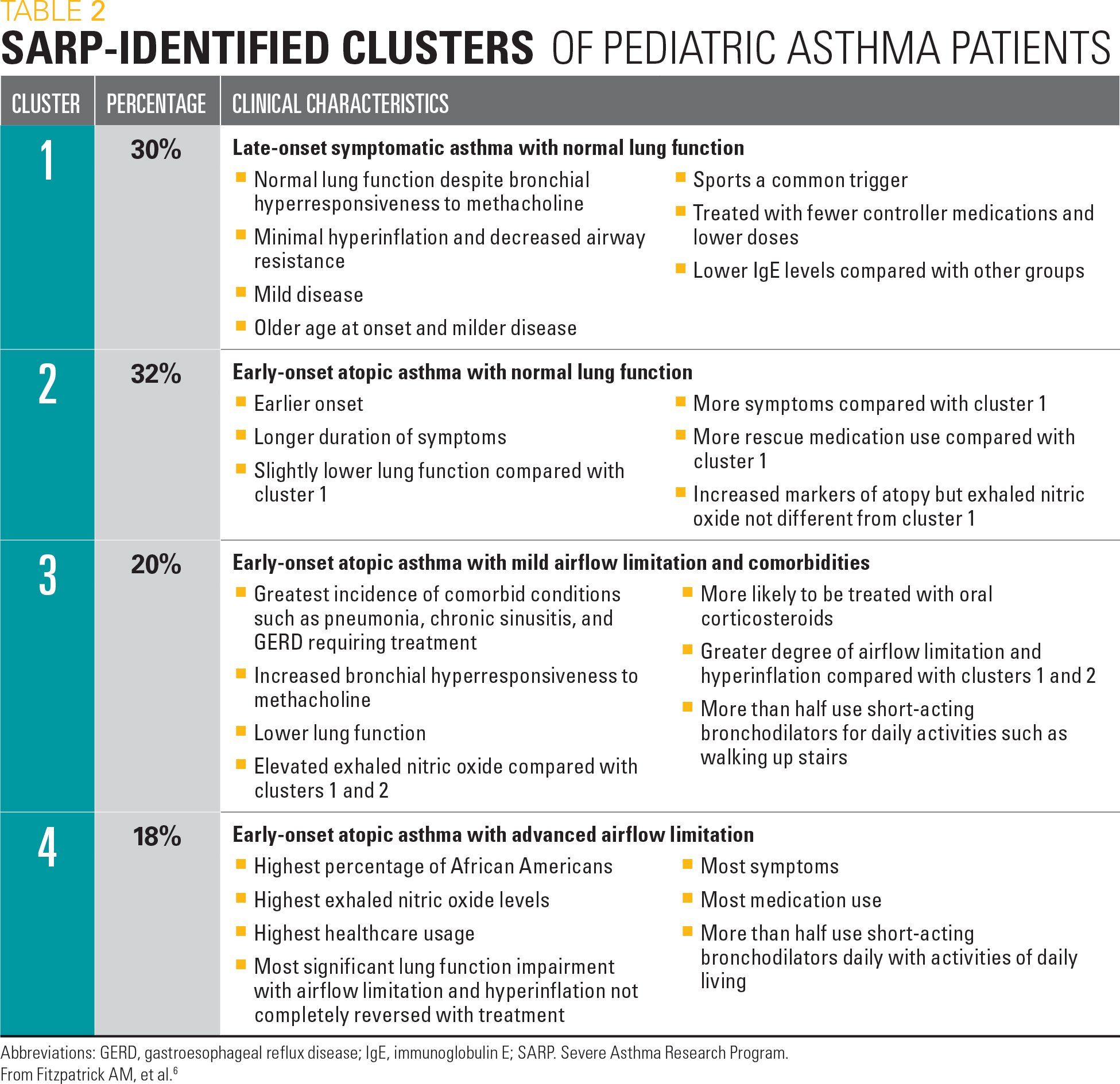
Table 2
ICD-10 codes for diagnosing asthma and comorbidities

Asthma is a heterogeneous disease with subsets of children responding very differently to medication. Some children have very few limitations while others have significant limitations and decreased lung function. A small subset of children with asthma have severe or refractory asthma, which represents a group that is at risk for significant morbidity and is also responsible for significant healthcare utilization and costs. This article will look at the definitions, risk factors, and treatments for severe asthma.
Definition
The joint European Respiratory Society (ERS)/American Thoracic Society (ATS) Task Force defines severe asthma as treatment requiring either of the following:1
1. High-dose inhaled corticosteroids (ICS, as defined here2) and a second controller medication over the last year.
2. Systemic corticosteroid treatment for at least half the prior year to prevent uncontrolled asthma or remaining uncontrolled despite the therapy.
The World Health Organization (WHO) identifies 3 specific groups that encompass severe asthma, each with its own challenges and treatment issues:3
· Untreated severe asthma.
· Difficult-to-treat asthma.
· Treatment-resistant asthma that is not controlled on high-dose steroids.
Uncontrolled vs severe asthma
Asthma is a complicated chronic illness and management is impacted by many domains. Whereas most asthmatic children can achieve good symptom control by avoiding triggers and regularly using controller medications, some will not achieve optimal results even with maximal therapy.4 One of the goals of the pediatrician is to identify whether a patient on maximal therapy has asthma refractory to treatment or whether symptoms are due to noncompliance, psychosocial factors, persistent environmental triggers, or comorbidities. Table 1 gives the pediatrician areas to examine to identify poorly controlled or undertreated asthma.
Clinical features and risk factors
Children with severe asthma are more likely to have received multiple courses of systemic corticosteroids, visited the emergency department (ED), or experienced a hospital admission for an asthma exacerbation in the prior 12 months. There is concern for an increased risk of asthma mortality in this group as nearly 1 in 3 have a history of intubation for near-fatal respiratory failure.2
Compared with children with nonsevere asthma and adults with severe asthma, elevated serum immunoglobulin E (IgE) and increased eosinophil levels are common. Children with severe asthma are also noted to have increased concentrations of exhaled nitric oxide and a different pattern of aeroallergen sensitization compared with children with nonsevere asthma. Sensitization to pollens and molds is more common in nonsevere asthma while sensitization to indoor allergens is more common among children with severe asthma. Finally, children with severe asthma demonstrate less airflow limitation and air trapping as measured by pulmonary function testing compared with adults with severe asthma.2
Risk factors for severe asthma in children include gender, race, comorbid conditions, and environmental exposures, among others.2
GENDER
Severe asthma is more common in boys compared with girls and the manifestations of severe asthma also vary by gender. Sex hormones are hypothesized to be the cause of some of these differences.
Examples for boys include:2
· Experiencing more airflow obstruction and incomplete reversal with bronchodilators.
· Have greater amounts of air trapping.
RACE
Children with severe asthma are more likely to be African Americans. Although it is unclear if this is related to centers doing this research being located in large urban centers, there is some research in adults indicating asthma among African Americans is phenotypically different.5
African Americans with severe asthma are more likely to have a greater body mass index and more airway obstruction compared with Caucasians with severe asthma. Further, serum IgE levels, allergy testing, and family history were all significantly associated with severe asthma in African Americans, but the same association was not present in Caucasians. The relationship between race and asthma severity is complex and remains an area of active research.5
COMORBID CONDITIONS
Comorbid conditions that the pediatrician commonly evaluates in patients with nonsevere asthma are also more frequent in children with severe asthma:2
· Gastroesophageal reflux.
· Sinopulmonary infections.
· Pneumonia requiring antibiotic therapy.
· Obesity.
Whether obesity in children with asthma results from decreased activity or increased exposure to corticosteroids or some other physiologic mechanism is unclear.2
ENVIRONMENTAL EXPOSURES
Although not studied specifically in severe asthma, environmental tobacco smoke (ETS) is associated with poor asthma control and increased risk of hospitalization. In adults, ETS is associated with increased damage to lungs, decreased levels of antioxidants in the lungs, and other pathophysiologic changes (eg, increased proliferation of epithelial cells, increased apoptosis, and decreased cell death suppression) that can lead to increased airway hyper-responsiveness and use of short-acting bronchodilators.2
Clusters of severe asthma
The National Heart, Lung, and Blood Institute Severe Asthma Research Program, or SARP, is a National Institutes of Health Research group that has better differentiated severe versus nonsevere asthma through phenotypic and genetic analysis. Children included in this evaluation are from 5 academic-affiliated centers, have difficult-to-control asthma, and are representative of patients that primary care pediatricians refer for specialty care.
Through characterization procedures that include questionnaires, spirometry, skin testing, blood testing such as IgE levels, and methacholine testing, SARP identified 3 variables (asthma duration, the number of asthma controller medications, and baseline forced expiratory volume (FEV) 1 percent predicted values) that were able to cluster pediatric asthma patients into 4 groups. See Table 2 for these classifications. Further emphasizing the heterogeneity of asthma, severe asthma was identified in all 4 clusters. Identifying such “phenotypes” allows for the potential of tailoring treatment strategies toward the unique characteristics of that group’s pathophysiology. Of concern to the pediatrician, these phenotypes cross definitions of asthma severity in the current asthma guidelines.6
These different asthma phenotypes allow for investigation of specific differences and provide more potential for a personalized medicine approach. One such pathway is the type 2 or atopic pathway that involves production of IgE and release of cytokines, such as interleukin (IL)-4, IL-5, and IL-13. The clinician is able to look at specific biomarkers for this pathway including blood and sputum eosinophilia, fractional exhaled nitric oxide (FeNO) levels, and serum periostin levels that are usually elevated in patients with severe asthma. Blood eosinophils and exhaled nitric oxide are readily available to the clinician, whereas sputum eosinophils take laboratory preparation and careful analysis, and serum periostin can vary with normal growth in children and is a specialized laboratory test. Treatments for severe asthma in specific patients are often targeted to these cytokines or their receptors.7
Current biologics
Biologics target proteins and receptors that lead to inflammation and produce asthma symptoms. Current biologics target components of the pathophysiology such as IgE and IL-5, as well as IL-4 and IL-13. Currently available biologics approved for pediatric patients include omalizumab, mepolizumab, benralizumab, and dupilumab.
OMALIZUMAB
Omalizumab is a monoclonal antibody.
Omalizumab decreases the release of inflammatory mediators related to the allergic response and lowers IgE levels by:8
· Binding to free IgE (but not IgE bound to mast cells).
· Inhibiting IgE from binding to mast cells, basophils, and dendritic cells.
· Down regulating IgE receptors.
· Decreasing mediator release.
· Decreasing allergic inflammation.
Omalizumab is indicated for children who:
· Are aged 6 years and older.
· Experience moderate-to-severe persistent asthma.
· Have inadequately controlled symptoms with an ICS.
· Have a positive skin prick test or in vitro reactivity to perennial aeroallergens.
Real-life, long-term treatment is safe in most patients with infrequent adverse effects. In 1 study, 91 patients were followed for as long as 9 years and received a total of 10,472 injections.9 Fifty-nine patients were treated for 3 to 9 years, including 14 whose treatment exceeded 6 years. Eighteen patients dropped out in the first year for reasons unrelated to treatment. Four patients reported adverse effects of rhinitis/conjunctivitis, injection site reaction, fatigue, or thrombosis but continued treatment. Six patients dropped out for treatment-related adverse effects, including arthralgia/myalgia (3 patients), urticaria, angioedema (1 patient), metrorrhagia (1 patient), and relapsing herpes labialis (1 patient).
Anaphylaxis was not reported in this study. There was 1 case of a cancer (gastrointestinal stromal tumor [GIST] of the stomach) diagnosed 1 year after starting treatment, but it was felt to be unrelated, and omalizumab was restarted. No relapse was seen with 3 years of treatment. Four additional patients had tumors prior to initiating omalizumab-9 (2 patients), 6, and 2 years-and none experienced a recurrence.9
Omalizumab has primarily been studied in atopic children with aeroallergen sensitization and IgE levels between 30 and 1300 IU/mL. Dosing occurs every 2 to 4 weeks, is weight based, and is dependent on pretreatment IgE levels.
A Cochrane review of omalizumab use in both adults and children found improved outcomes compared with placebo in terms of:10
· Absolute reduction of asthma exacerbations from 26% to 16%.
· Decreased asthma exacerbations (odds ratio [OR], 0.55; 95% confidence interval [CI], 0.42-0.60).
· Decreased hospitalizations (OR, 0.16; 95% CI, 0.06-0.42).
· Increased likelihood of withdrawing ICS therapy (OR, 2.50; 95% CI, 2.00-3.13).
· Small decreases in rescue inhaler use (mean difference (MD), -0.39 puffs per day; 95% CI, -0.55– -0.24).
The review, however, showed no significant difference in the number of patients able to withdraw completely from oral corticosteroids (OR, 1.18; 95% CI, 0.53-2.63). Severe asthmatics who were on both inhaled and systemic corticosteroids saw no decreases in rescue inhaler use.10
Randomized controlled trials have also shown benefits of omalizumab. In the National Institute of Allergy and Infectious Diseases (NIAID) Inner-City Anti-IgE Therapy for Asthma (ICATA) trial, 419 atopic patients aged 6 to 20 years with primarily moderate to severe asthma were randomized to 60 weeks of omalizumab or placebo. Treatment reduced asthma symptoms by nearly 25%, decreased exacerbations from 49% to 30%, and allowed for reductions in both inhaled glucocorticoids and long-acting beta-agonists.11
In the NIAID Preventative Omalizumab or Step-up Therapy for Severe Fall Exacerbations (PROSE) study, 513 inner-city asthmatic children aged 6 to 17 years with a recent asthma exacerbation were randomized to 1 of 3 arms for 4 months (1 month prior to starting school and the following 3-month period) after a 4- to 9-month period of guideline-based therapy.
The objective of the study was to prevent fall asthma exacerbations and included the following treatment arms:12
· Omalizumab (with inhaled placebo).
· ICS boost (with injected placebo).
· Guidelines-based care with injected placebo and inhaled placebo.
Patients already at the maximal ICS dose were not enrolled in the ICS boost arm. Exacerbation rates were significantly lower in the omalizumab arm compared with placebo (11.3% vs 21.0%, respectively; OR, 0.48; 95% CI, 0.25-0.92) but not to the ICS boost arm (8.4% vs 11.1%; OR, 0.73; 95% CI, 0.33-1.64). In those patients already at high-dose ICS and not eligible for the ICS boost treatment arm, omalizumab decreased exacerbations by more than 50% (32.6% vs 15.1%). Interestingly, omalizumab primarily benefited patients having an exacerbation during the 4- to 9-month run-in phase of the study but was not beneficial for those that did not have an exacerbation during this time period. The investigators discussed a possible seasonal strategy to prevent fall asthma exacerbations for patients with severe asthma that were not eligible for ICS boost in PROSE, or patients of any severity with a recent exacerbation to provide a more targeted seasonal approach to management for those susceptible to recurrent fall asthma exacerbations as a preventive strategy.12
MEPOLIZUMAB
Through its role in the production, differentiation, maturation, recruitment, and activation of eosinophils, IL-5 is a major contributor to allergic inflammation. Mepolizumab is a monoclonal antibody against IL-5 and inhibits the production, maturation, proliferation, release from bone marrow, and activation of eosinophils.8
Mepolizumab is given via subcutaneous injection every 4 weeks and is indicated in patients aged older than 12 years with severe persistent asthma with eosinophilic asthma.
Mepolizumab has a low incidence of adverse effects, but the most common are:8
· Asthma exacerbation.
· Headaches.
· Injection site reaction.
· Back pain.
· Weakness.
· Fatigue.
· Oropharyngeal pain.
· Bronchitis/upper respiratory tract
· infection.
· Severe allergic reactions (rare).
A Cochrane review of anti-IL-5 therapies for asthma use in both adults and children found mepolizumab improved outcomes compared with placebo in terms of:13
· Decreased rate of asthma exacerbation (rate ratio [RR], 0.45 [0.36-0.55]).
· Decreased rate of asthma exacerbations requiring ED treatment or hospital admission (RR, 0.52 [0.31-0.87]).
· Significant improvements in prebronchodilator FEV1 (mean difference [MD], 0.08; 95% CI, 0.02-0.15).
There was no significant change in health-related quality of life. Adverse events leading to discontinuation of mepolizumab occurred less commonly in treated patients compared with placebo (19/1000 and 26/1000, respectively; RR, 0.72 [0.18-2.92]).13
The Dose Ranging, Efficacy, and Safety with Mepolizumab (DREAM) study randomized 621 asthmatics that included both adults and children to 3 different doses of mepolizumab or placebo. The DREAM study found a reduction in asthma exacerbations, ED visits, and hospital admissions.7
BENRALIZUMAB
Benralizumab is a monoclonal antibody against the IL-5 receptor alpha that is found on the surface of basophils and eosinophils.8 It is approved for use in children aged 12 years and older.
The most common adverse effects include:8
· Nasopharyngitis.
· Injection site reaction.
· Upper respiratory tract infection.
· Worsening asthma.
In 1 study, a small number of patients were also noted to have fever, tachycardia, and anxiety.8
A Cochrane review of anti-IL-5 therapies for asthma use in both adults and children found benralizumab improved outcomes compared with placebo in terms of:13
· Decreased rate of asthma exacerbation (0.69; 95% CI, 0.56-0.85).
· Decreased rate of asthma exacerbations requiring ED treatment or hospital admission (0.68 [0.47-0.98]).
· Improvement in health-related quality of life (MD, 0.23 units; 95% CI, 0.11-0.35 units). Change of more than 0.5 units is considered clinically significant.
· Significant improvements in prebronchodilator FEV1 (MD, 0.10 L; 95% CI, 0.05-0.14 L).
· Adverse events leading to discontinuation of benralizumab occurred more commonly in treated patients compared with placebo (19/1000 and 9/1000, respectively; RR, 2.15 [1.02-4.57]).13
DUPILUMAB
Dupilumab is a human monoclonal antibody to the alpha subunit of the IL-4 receptor, thereby blocking the activity of IL-4 and IL-13. It was approved for the treatment of moderate-to-severe asthma in children aged 12 years and older and adults as add-on maintenance therapy. It is administered every 2 weeks via the subcutaneous route. A study in adolescents noted a 47% reduction in asthma exacerbations as well as an increase in pulmonary function. It was previously prescribed for the treatment of severe atopic dermatitis in adults.14
Summary
The pediatrician needs to be aware of how to identify poorly controlled asthma that does not respond to conventional management. There are biologics available to treat these patients and more in the pipeline that the pediatrician may see following referral to an asthma specialist.
References:
1. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation, and treatment of severe asthma. Eur Respir J. 2014;43(2):343-373.
2. Fitzpatrick AM. Severe asthma in Children: lessons learned and future directions. J Allergy Clin Immunol Pract. 2016;4(1):11-9.
3. Bousquet J, Mantzouranis E, Cruz AA, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126(5):926-938.
4. Bateman ED, Boushey HA, Bousquet J, et al; GOAL Investigators Group. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit CareMed. 2004;170(8):836-844.
5. Gamble C, Talbott E, Youk A, et al. Racial differences in biologic predictors of severe asthma: data from the Severe Asthma Research Program. J Allergy Clin Immunol. 2010;126(6):1149.e1-1156.e1.
6. Fitzpatrick AM, Teague WG, Meyers DA, et al; National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127(2):382-389.e1-13.
7. Abrams EM, Becker AB, Szefler SJ. Current state and future of biologic therapies in the treatment of asthma in children. Pediatr Allergy Immunol Pulmonol. 2018;31(3):119-131.
8. Agumadu VC, Ramphul K, Mejias SG, Sonaye R, Sombans S, Lohana P. A review of three new anti-interleukin-5 monoclonal antibody therapies for severe asthma. Cureus. 2018;10(8):e3216.
9. Di Bona D, Fiorino I, Taurino M, et al. Long-term “real-life” safety of omalizumab in patients with severe uncontrolled asthma: a nine-year study. Respir Med. 2017;130:55-60.
10. Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
11. Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005-1015.
12. Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476-1485.
13. Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834.
14. Abrams EM, Becker AB, Szefler SJ. Current state and future of biologic therapies in the treatment of asthma in children. Pediatr Allergy Immunol Pulmonal. 2018;31(3):119-131.

Having "the talk" with teen patients
June 17th 2022A visit with a pediatric clinician is an ideal time to ensure that a teenager knows the correct information, has the opportunity to make certain contraceptive choices, and instill the knowledge that the pediatric office is a safe place to come for help.