Infant With Recurrent Omphalitis and Otitis
A baby boy, aged 14 days, presented with a temperature of 38.2°C (100.8°F), a generalized maculopapular rash, and purulent otorrhea. He was treated with oral amoxicillin for 10 days. At age 25 days, he again presented-this time with erythema and edema of the umbilicus, thrush, and fever of 24 hours’ duration.
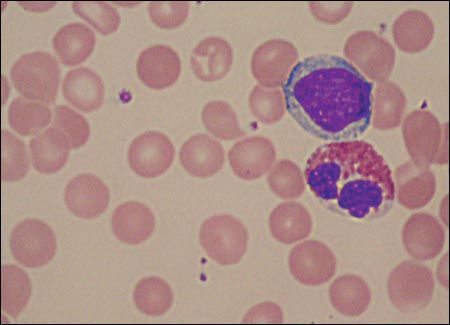
Figure 1 – This peripheral blood smear shows a lymphocyte on top of an eosinophil; no neutrophils are identified.
A baby boy, aged 14 days, presented with a temperature of 38.2°C (100.8°F), a generalized maculopapular rash, and purulent otorrhea. He was treated with oral amoxicillin for 10 days. At age 25 days, he again presented-this time with erythema and edema of the umbilicus, thrush, and fever of 24 hours' duration. The cord stump had dried and fallen off 2 days earlier. (The umbilical stump had been treated with triple dye in the newborn nursery, and there was no history of umbilical catheterization.) He was admitted to the local hospital for parenteral antibiotic treatment of the omphalitis. Purulent fluid draining from the umbilicus grew Pseudomonas aeruginosa, Enterobacter cloacae, and Enterococcus faecalis. An abdominal ultrasonogram revealed a fluid collection in the retro-umbilical area. After completion of 14 days of intravenous imipenem/cilastatin, which had been started empirically, both the omphalitis and the drainage of retro-umbilical fluid had resolved.
One week after completion of antibiotic therapy, the omphalitis and fever recurred, and the patient was again admitted for parenteral antibiotic therapy with imipenem/cilastatin. At this time, a sepsis evaluation with lumbar puncture was performed. Findings on cultures of urine, blood, and cerebrospinal fluid were unremarkable. However, draining umbilical fluid grew Citrobacter amalonaticus, as well as the organisms previously identified. Again, a retro-umbilical fluid collection was noted on ultrasonography. This time, the abscess was incised and drained. On day 6 of this hospitalization, otorrhea once more developed and was treated with ciprofloxacin otic. Cultures from the infant's right ear grew Staphylococcus epidermidis. He was discharged after 10 days, with complete resolution of the infections. At age 62 days, he returned to his primary care physician with erythema of the umbilicus. This time, he was transferred to a regional children's hospital for further evaluation and treatment.
The patient was born at term, via uncomplicated repeat cesarean delivery, to a 25-year-old, gravida 3 mother. His mother had gestational diabetes, which was treated with insulin. The patient received his first hepatitis B vaccination at birth and had received his 2-month immunizations just before referral. The patient lives with both parents, a 3-year-old sister, and a 6-year-old sister, all of whom are healthy. He does not attend day care. There are no unusual exposures at home.
Physical examination on arrival at the children's hospital showed an afebrile, well-appearing infant. He had fed vigorously throughout his illnesses and appeared well-nourished. He was at the 50th percentile for height, weight, and head circumference. There were no rashes. The umbilicus was nontender and was not draining; there was a surrounding 2 × 1-cm area of dull erythema without induration. Examination of the ears, nose, and throat revealed purulent fluid draining from both ears. There was no lymphadenopathy. No cardiorespiratory irregularities were noted. The abdomen was soft and nondistended with normal bowel sounds. There were no genitourinary or neurological abnormalities.
A complete blood cell count on admission revealed a hemoglobin level of 10.3 g/dL; platelet count, 385,000/μL; and leukocyte count, 13,800/μL, with a differential of 0% neutrophils, 72% lymphocytes, 17% monocytes, and 11% eosinophils. A peripheral smear confirmed a lack of neutrophils but was otherwise unremarkable (Figure 1). C-reactive protein level was 1.0 mg/dL (normal, 0.0 to 0.7 mg/dL). Chemistry panel results were significant for mild elevations of aspartate aminotransferase and alanine aminotransferase levels (81 U/L and 62 U/L, respectively). After the discovery of severe peripheral neutropenia, laboratory results from the referring hospital were reviewed; these revealed that the patient had a white blood cell count of 10,000/μL and an absolute neutrophil count (ANC) of 0/μL when he was first hospitalized at age 21 days.
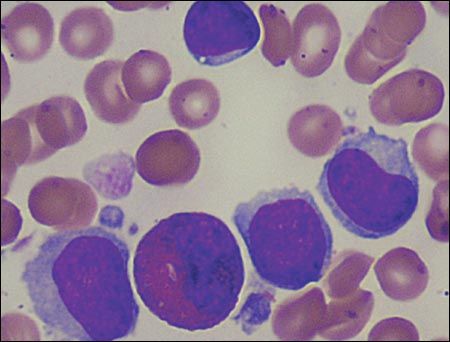
Figure 2 – In this bone marrow sample, 4 lymphocytes and 1 eosinophil are evident; neither myeloid precursors nor differentiated myeloid elements (bands, segmented neutrophils) are identified.
During his stay at the children's hospital, several pediatric subspecialists, including specialists in infectious disease, hematology/oncology, and rheumatology, evaluated the patient and ordered additional studies. A bone marrow sample was normocellular with myeloid hypoplasia and myeloid maturational arrest (Figure 2). Epstein-Barr virus (EBV) titers were positive for viral capsid antigen (VCA) IgG and negative for VCA IgM. Cytomegalovirus (CMV) titers and results of a polymerase chain reaction test for HIV infection were negative. Quantitative immunoglobulin studies revealed an IgG level of 757 mg/dL (normal, 198 to 577 mg/dL) and an IgM level of 153 mg/dL (normal, 16 to 100 mg/dL). T- and Blymphocyte assays were significant for elevations of CD4 (twice normal), CD56 (4 times normal), and CD19 (5 times normal). No antineutrophil antibodies were detected. There was a normal male chromosome complement, and results of an osseous survey were normal.
WHAT IS GOING ON?
It seemed likely that the patient's severe neutropenia underlay his recurring episodes of omphalitis. The differential diagnosis of severe neutropenia in a newborn includes severe congenital neutropenia; acquired primary bone marrow failure, possibly of viral origin (eg, secondary to HIV, EBV, or CMV infection); severe combined immunodeficiency; agammaglobulinemia; defects of neutrophil adhesion or function; neutropenias associated with metabolic or immunological disorders, such as autoimmune neutropenia of infancy; and neutropenia as one feature of a complex syndrome, such as Shwachman-Diamond syndrome, dyskeratosis congenita, Fanconi anemia, or Chédiak-Higashi syndrome.1
However, other possible causes of recurrent omphalitis were also considered. Either a resistant organism that had not been eradicated or incomplete treatment of the retroumbilical abscess could explain recurrence of the umbilical infection. In addition, we considered the possibility of an abnormal umbilicus, for which the differential diagnosis includes retained umbilical cord remnants, a granuloma, umbilical polyps, and patency of urachal or omphalomesenteric ducts causing fistulas, tracts, or cysts that are prone to infection.
The patient's blood cell counts and bone marrow sample confirmed the diagnosis of severe congenital neutropenia.
SEVERE CONGENITAL NEUTROPENIA: OVERVIEW
The incidence of severe congenital neutropenia (also called simply "congenital neutropenia") is about 1 or 2 cases per million.2 Males and females are affected in equal numbers. The initial clinical features of severe congenital neutropenia include early bacterial infections of the skin and mucosae (eg, omphalitis and paronychias in the neonatal period; recurrent upper and lower respiratory tract infections, sepsis, and failure to thrive in older children).1
Severe congenital neutropenia was first reported in 1956 by Rolf Kostmann, a Swedish pediatrician.1 At the time of Kostmann's report, the agranulocytosis he described as being the causative mechanism was thought to be an acquired condition resulting from toxins or infectious exposures with subsequent bone marrow damage.1 After diligent investigation of medical and histological records as well as patterns of inheritance, Kostmann concluded that the disorder was caused by a single recessive autosomal gene. Today, severe congenital neutropenia is characterized as a group of heterogeneous hematological disorders with different patterns of inheritance, including autosomal recessive, autosomal dominant, and sporadic occurrences.2 The disorder now known as classic Kostmann syndrome is severe congenital neutropenia that has an autosomal recessive inheritance pattern.
Laboratory findings consistent with the diagnosis of severe congenital neutropenia include an ANC of less than 500/μL, mild anemia, thrombocytosis, eosinophilia, monocytosis, and elevated IgG levels. Bone marrow examination reveals maturational arrest of myelopoiesis with depletion of mature neutrophils.1 Later in the disease course, clinical findings may include periodontitis, hepatosplenomegaly, osteoporosis, and secondary malignancies. The cause of the later clinical features is not well understood; they may result from the chronic inflammation of the disease process, an underlying deficiency of antibacterial peptides, or lifelong therapy with recombinant granulocyte colony-stimulating factor (G-CSF).
Most of the patients with severe congenital neutropenia who were initially described by Kostmann died of serious infections in spite of antibiotic therapy.3,4 Bone marrow transplantation was the only treatment option until the late 1980s, when recombinant G-CSF became available.5 The use of G-CSF has markedly improved the clinical outcomes and quality of life for children with severe congenital neutropenia. Data from the Severe Chronic Neutropenia International Registry (note that severe congenital neutropenia is a type of severe chronic neutropenia) show that more than 90% of patients treated with G-CSF have responded with an increased ANC of greater than 1000/μL, fewer infections requiring antibiotics, and fewer hospitalizations.3,4,6
Nonetheless, some authorities have expressed concerns regarding the long-term use of G-CSF because of the perceived increased risk of secondary malignancies.5,6 Although the mechanisms underlying the evolution of secondary malignancies in patients receiving G-CSF are not completely understood, the fundamental stem cell defect seen in severe congenital neutropenia has been suggested as a cause.3,7 Since the use of G-CSF therapy began, the incidence of malignant myeloid disorders in treated patients has been about 10%, with a cumulative incidence of 21% over 10 years.4,5 (Comparisons with the pre-G-CSF era are difficult because many patients from that time did not survive infancy.) Still, G-CSF remains the firstline treatment for most patients. For those who do not respond to G-CSF, hematopoietic stem cell transplant is the only viable option. If treatment with hematopoietic stem cell transplant is successful, the patient's peripheral blood counts normalize and G-CSF treatments are no longer needed.5
IS NEUTROPENIA ALONE TO BLAME?
Although this patient clearly had severe neutropenia, it seemed suspicious that his umbilical cultures continued to grow GI flora. Another abdominal ultrasonogram was obtained to explore the possibility of an intestinal connection with the umbilicus. No such connection was found, nor was there any evidence of retro-umbilical fluid. Subsequently, a voiding cystourethrogram was completed to evaluate for a patent urachus. The patient was found to have grade II vesicoureteral reflux on the right and grade I on the left-but there was no evidence of a persistent urachus. Finally, exploratory surgery was performed, which revealed a patent omphalomesenteric duct; the remnants of the duct were removed from its origin in the ileum.
Thus, while neutropenia had created an environment for opportunistic infections in our patient, the underlying reason for his recurring polymicrobial omphalitis was a persistent omphalomesenteric duct.
PERSISTENT OMPHALOMESENTERIC DUCT: AN OVERVIEW
"Yolk stalk," "vitelline duct," and "omphalomesenteric duct" are synonymous terms that identify the duct that arises from the yolk sac and transfers nutrients to the developing embryo during early gestation. Once the placenta is well established, the sac atrophies and the stalk normally detaches from the midgut by week 6.8 Partial or complete failure of involution results in various residual structures that can form fistulas, sinuses, cysts, or diverticula.9 In 2% of patients, the proximal portion of the yolk stalk remains patent, forming a Meckel diverticulum.10 In this patient's case, the entire duct remained patent from the distal ileum to the external umbilicus, a finding referred to as a persistent omphalomesenteric duct.
Overall, vitelline duct remnants are very rare, with Meckel diverticulum being by far the most common type. The usual presentation of a persistent omphalomesenteric duct is fecal umbilical drainage; however, persistent infection (as in this patient), intestinal obstruction, intussusception through the duct, and prolapse of the duct leading to polyps have also been reported.9,11 The recommended diagnostic imaging is fluoroscopy or sinography.12 Treatment requires surgical excision of the duct remnants.9
OUTCOME OF THIS CASE
This infant had recurrent, polymicrobial omphalitis secondary to a persistent omphalomesenteric duct; the omphalitis was exacerbated by severe congenital neutropenia. A 10-day course of intravenous meropenem was completed before discharge from the children's hospital; the antibiotic therapy once more resulted in full resolution of the omphalitis and otitis. Treatment with GCSF was started in an effort to encourage neutrophil production. The patient has been followed in the hematology/oncology outpatient clinic; he has had a poor response to the G-CSF despite repeated dosage increases. Results of a second bone marrow biopsy, 3 months after his initial study, were unchanged from those seen with the first sample. Most recently, at 6 months of age and after 4 months of daily G-CSF injections, the patient's ANC was 410/μL.
References:
REFERENCES:
1.
Carlsson G, Andersson M, Pütsep K, et al. Kostmann syndrome or infantile genetic agranulocytosis, part one: celebrating 50 years of clinical and basic research on severe congenital neutropenia.
Acta Paediatr
. 2006;95:1526-1532.
2.
Zeidler C, Schwinzer B, Welte K. Congenital neutropenias.
Rev Clin Exp Hematol
. 2003;7:72-83.
3.
Carlsson G, Melin M, Dahl N, et al. Kostmann syndrome or infantile genetic agranulocytosis, part two: understanding the underlying genetic defects in severe congenital neutropenia.
Acta Paediatr
. 2007;96:813-819.
4.
Welte K, Zeidler C, Dale DC. Severe congenital neutropenia.
Semin Hematol
. 2006;43:189-195.
5.
Skokowa J, Germeshausen M, Zeidler C, Welte K. Severe congenital neutropenia: inheritance and pathophysiology.
Curr Opin Hematol
. 2007;14:22-28.
6.
Kaushansky K. Lineage-specific hematopoietic growth factors.
N Engl J Med
. 2006;354:2034-2045.
7.
Carlsson G, Aprikyan AA, Ericson KG, et al. Neutrophil elastase and granulocyte colony-stimulating factor receptor mutation analyses and leukemia evolution in severe congenital neutropenia patients belonging to the original Kostmann family in northern Sweden.
Haematologica
. 2006;91:589-595.
8.
Moore KL, Persaud TV. The placenta and fetal membranes. In:
The Developing Human: Clinically Oriented Embryology
. 8th ed. Philadelphia: Saunders; 2008:110-144.
9.
Pomeranz A. Anomalies, abnormalities, and care of the umbilicus.
Pediatr Clin North Am
. 2004;51: 819-827, xii.
10.
Richardson LS, Singer JI, Buchino JJ. Painful, red umbilicus.
Pediatr Emerg Care
. 2004;20:547-550.
11.
Saad DF, Gow KW. Images in clinical medicine. Small-bowel prolapse through a persistent omphalomesenteric duct.
N Engl J Med
. 2005;353:e1.
12.
Snyder CL. Current management of umbilical abnormalities and related anomalies.
Semin Pediatr Surg
. 2007;16:41-49.
Recognize & Refer: Hemangiomas in pediatrics
July 17th 2019Contemporary Pediatrics sits down exclusively with Sheila Fallon Friedlander, MD, a professor dermatology and pediatrics, to discuss the one key condition for which she believes community pediatricians should be especially aware-hemangiomas.