Snippets of Vaccine History: Success, Failure, and Controversy
Vaccination against infectious diseases has saved millions of lives. The recurrent threat of influenza pandemics and the prevalence of global HIV infections underscore the need for better-designed, more effective vaccines.
Vaccination against infectious diseases has saved millions of lives. The recurrent threat of influenza pandemics and the prevalence of global HIV infections underscore the need for better-designed, more effective vaccines. The following historical vignettes illustrate that a vigorous, sustained global vaccination campaign can eradicate ancient diseases, such as smallpox. Conversely, even a well standardized live-attenuated BCG vaccine may be ineffective when given to a population with different ethnicity, genetic background, nutritional status, and exposure to nonpathogenic environmental mycobacteria. Because injected attenuated live organisms may cause adverse effects, such as pain and fever, and rare dangerous complications, the new trend is to produce genetically engineered subunit vaccines. These new vaccines use only a targeted portion of the whole organism and can potentially improve immunization potency and lessen adverse effects.
Historically, people were wary of being vaccinated with material prepared in animal tissues. Accidental contamination by an irrelevant virus of the vaccinating preparation also caused anxiety and resistance. Today, the media, Internet, and patient/parent advocacy can negatively influence public opinion, resulting in refusal of vaccination. This can lead to increased morbidity and mortality among unvaccinated children, as occurred in England. Thus, it is the task of public health agencies, physicians, and pediatricians to provide clear, persuasive information on the safety, efficacy, and necessity of routine vaccinations.
THE PIONEER OF SMALLPOX VACCINATION
Edward Jenner (1749-1823), a British country doctor, is widely credited by posterity as the pioneer of anti-smallpox vaccination. In 1796, he inoculated an 8-year-old boy with material obtained from the lesion on the udder of a cow infected with cowpox. The rationale was based on rural folklore that milkmaids who became infected with cowpox never contracted smallpox, which had been endemic in Britain. The boy indeed became resistant to smallpox. Jenner established the principle of vaccination (derived from vacca, Latin for cow) and introduced the idea of induced cross-immunity.
For his discovery and tireless promotion of the practice of vaccination, Jenner twice received monetary prizes from the British Parliament. Yet the real pioneer of vaccination was a farmer from Dorsetshire named Benjamin Jesty (1737-1816). As a child, he had been infected with cowpox and knew about the belief of life-long resistance to smallpox. In 1774, 22 years before Jenner’s inoculation, Jesty took a darning needle dipped in pustular material from a cow’s udder and scratched the arms of his wife and 2 sons. They had a brief illness, recovered, and never contracted smallpox.
As rumor spread about the inoculation, other farmers responded with hostility. They were repulsed by the idea of being injected with bovine material; some feared “turning into a horned beast.”1 Nevertheless, in 1805 before 12 officials of the original Vaccine Pock Institution in London, Jesty presented his grown-up son who had never had smallpox as proof of the efficacy of his inoculation. Pearson, the founder of the Institution, was sufficiently convinced and presented evidence on Jesty’s success before the House of Commons in 1807. Although he received no reward from Parliament, the Institution gave him a pair of gold-mounted lancets, a testimonial scroll, and 15 guineas for expenses. It also commissioned to have his portrait painted. Jesty insisted that he be portrayed in his farmer’s clothes. He proved to be a fidgety sitter but quieted down when the artist’s wife played the piano. After a long search, his portrait was found and is now displayed together with Jenner’s portrait in the Wellcome Library in London (Figure 1).2

THE ERADICATION OF SMALLPOX
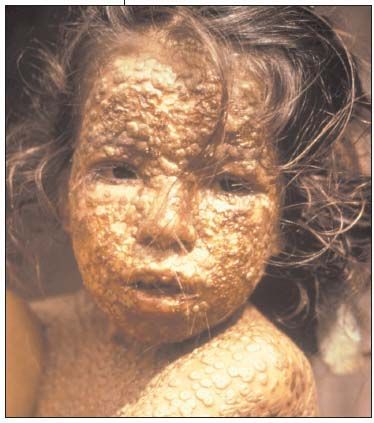
Smallpox was an ancient scourge in several continents. The mummy of the pharaoh Ramses V bore the signs of pockmarks on his face. The periodic global epidemics of smallpox were accompanied by 20% to 40% mortality among the unvaccinated; the survivors were disfigured and some became blind (Figure 2). Mass vaccinations with cowpox successfully eradicated the disease in Western Europe, North America, and Japan. Yet in 1967, 10 to 15 million cases were recorded in countries where the disease was endemic. In the same year, the WHO initiated the Intensified Smallpox Eradication Program in the affected countries.3 The goal, based on the success of previous campaigns, was to mass vaccinate 100% of the population. This was because of a report in 1964 stating that an 80% vaccination rate in India still left pockets of persistent infections. Interestingly, an outbreak in Western Nigeria, with more than 90% of the population already vaccinated, indicated that even such a high rate of vaccination was not protective against an outbreak. Therefore, a new strategy of surveillance and containment was devised by vaccination teams in the field.
In 1970, an outbreak of smallpox in southwestern India infected more than a thousand persons and caused more than 100 deaths. This called for a major mobilization of all health personnel who had to detect new cases among 2 million people. Their effort involved a house to house search for new cases, exact reporting of any new incidence, enforced isolation, and containment vaccination. The newly developed surveillance-containment strategy was so successful that smallpox was eliminated in this part of India within weeks.3 The rest of India slowly accepted the strategy. Ingenious field teams often improvised procedures to adapt to local conditions.
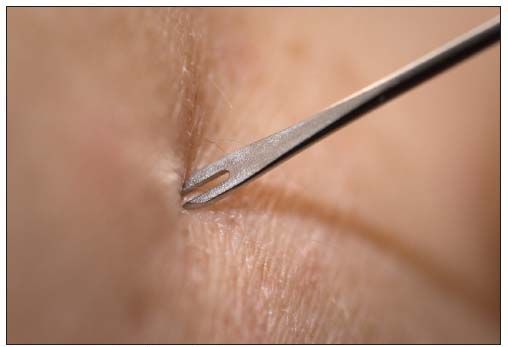
Success of this strategy was helped by the use of the following innovations: freeze-dried vaccines that extended shelf life, jet injectors that vaccinated more than 1000 persons per hour, and bifurcated needles for the skin scarification of vaccinees (Figure 3). Because of the meticulous collection of field data and the analysis of the efficacy of implemented changes, India, despite its size and geographical, political, and social diversity, successfully eradicated smallpox within 9 years. The last case of smallpox occurred in 1977 in Somalia. Smallpox became the first global infectious disease that was completely eradicated.3
FAILURE OF BCG VACCINATION IN INDIA
Tuberculosis (TB) caused by Mycobacterium tuberculosis is still a major infectious disease. Worldwide, it kills 2 million people annually. The incidence of the disease is enhanced by the global prevalence of HIV infection. The available prevention is provided by the BCG vaccine, first prepared almost 100 years ago. Albert Calmette and Jean Camille Gurin applied the same principle used for the preparation of smallpox vaccine-use of a cross-reactive organism, the virulence of which also had to be attenuated. Thus, a virulent strain of Mycobacterium bovis that caused TB in cows was isolated. The bacteria were notoriously difficult to grow in a dish. To facilitate growth and diminish clumping, Calmette and Gurin grew the organisms on potato slices with glycerol and ox bile as a detergent. The first isolates grew on the dish with changed morphology and reduced virulence when injected into guinea pigs.
With the intent of a human vaccine preparation and with remarkable perseverance, the researchers serially passaged the bacteria for 13 years. Attenuated virulence was proven by the failure of inducing lesions in a variety of experimental animals and full protection against a challenge with the virulent strain. In 1921, the vaccine was tested in infants and was found to be safe and protective against the disease. By 1929, 60 countries used BCG vaccination. In the ensuing decades, the WHO included the BCG vaccine in its Program of Immunization, and hundreds of millions were vaccinated.
Then a baffling picture emerged. The vaccine protected children against TB-meningitis and miliary (disseminated) TB; however, its efficacy in adults against the pulmonary form ranged between 0% and 80%.4 Low or absent protection was observed in Africa and India. An intensive search was launched to find the cause(s). The longevity of the continued in vitro passages still done in the laboratories was the most obvious suspected cause of the waning immunizing potency. This was compounded by the heterogeneity of the strains grown worldwide and the genetic background and age of the humans vaccinated. Some even proposed that the freeze-drying method for elimination of the serial passage of the microbes diminished the viability of the organisms. Years later, genomic studies showed that the various vaccinating strains contained gene deletions and duplications and had variable levels of virulence factors.
The failure in India of BCG vaccination was, in part, explained by the extensive exposure of humans to environmental nontuberculous mycobacteria from soil or grass; the mycobacteria caused infection in exposed persons and inhibited the development of an anti-BCG immune response. Another hypothesis was that nutritional and genetic differences in human populations could influence the efficacy of the BCG vaccine.3
Researchers are currently attempting to develop a more effective anti-TB vaccine using recombinant BCG organisms that overexpress the protective antigens, such as 85B of M tuberculosis.5 This new vaccine has been shown to be strongly immunogenic in experimental animals. Another method, “prime-boost,” first inoculates a person with the BCG vaccine and then administers various kinds of subunit vaccines (DNA, protein) to boost the potency of the primary response. Almost 100 years after its preparation, the search for an efficient and safe anti-TB vaccine continues.
SERENDIPITY THAT SAVED THE LIVES OF MILLIONS
In the 1950s, Baruch S. Blumberg, MD, became interested in the genetics of disease susceptibility. He collected sera from humans with different ethnic backgrounds. He reasoned that multitransfused persons had developed antibodies against donor serum proteins and used such sera to detect polymorphism, such as seen in blood group proteins and haptoglobins.4
After many trials, Blumberg found 1 serum from an Australian aborigine that gave a positive reaction, dubbed the Australia (Au) antigen. This led to the search for positive sera elsewhere. In the United States, very few normal sera gave a reaction; however, many sera from persons with Down syndrome and leukemia patients were positive. Significantly, a high percentage of sera from Asian persons were also positive.
No explanation could be found for the presence of the Au antigen until a lucky break provided an important clue. One of the Au-negative patients with Down syndrome became infected with viral hepatitis, and the Au antigen appeared in his serum. Blumberg hypothesized that the Au antigen is closely related to or is the etiologic agent of viral hepatitis. Moreover, Japanese researchers observed that Au-positive sera transfused into healthy persons caused hepatitis. The next step was clear. The immunoassay developed by Blumberg and his associates could now be used by blood banks to screen donor sera before transfusion to eliminate the Au-positive samples. Apart from the improved safety, this routine test saved a half billion dollars.
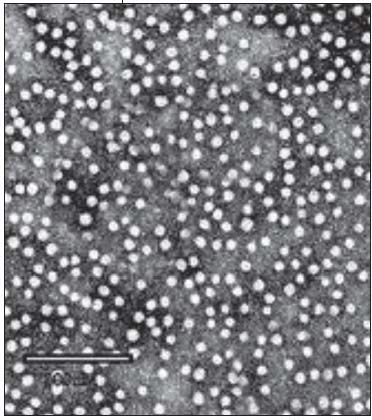
Subsequently, electron microscopic observation detected in the sera rod-shaped and spherical viral particles measuring 20 nm (Figure 4). These turned out to be the outer coat of the virus, later called the hepatitis B surface antigen (HBsAg). Blumberg and his associates realized that their initial accidental discovery had far-reaching implications. The more refined radioimmunoassay indicated that an enormous amount of viral antigen circulates in patient sera. They conceived the totally unconventional idea that the particles could be used for the preparation of a vaccine. This was feasible because the viral coat proteins were noninfectious. The particles were centrifuged from the sera and heat killed.6 Later, Merck used pepsin urea and formalin to purify and inactivate the preparation.
This vaccine was hugely effective in hundreds of millions of people in Asia and Africa and significantly decreased the rate of carriers of the disease and the incidence of liver cancer, a possible sequel to viral hepatitis. In the United States, clinicians were reluctant to use a vaccine prepared from the sera of infected persons. The vaccine was withdrawn from the market and, in 1988, was replaced by the hepatitis B vaccine prepared by the expression of the viral coat proteins in baker’s yeast. As an acknowledgment of his discovery, deemed one of the more important ones of the 20th century, Blumberg was awarded the 1976 Nobel Prize in Medicine.
CONTAMINATED POLIO VIRUS VACCINE
By 1952, Jonas Salk, MD, and his colleagues developed a vaccine against the 3 strains of the poliovirus. This became possible because the virus could be grown in rhesus monkey kidney cells and then inactivated by formalin. After successful pilot trials, the vaccine was declared safe and effective.7 In the ensuing years, about 2 million children were vaccinated in the United States, Canada, and Finland.
After receiving her PhD in bacteriology, Bernice Eddy (1903-1989) worked at the Division of Biological Standards of the NIH, where she was in charge of safety testing of the poliovirus vaccine used for children. Eddy and her colleague Sarah Stewart, MD, PhD (1906-1976), discovered a mouse virus, which when injected into baby hamsters caused tumor growth. They called it polyomavirus because it induced tumors in several animal species.
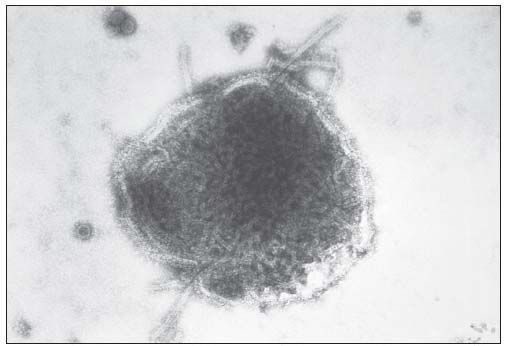
In 1960, Eddy, on her own initiative, decided to test the monkey kidney cells used for polio vaccine preparation. Injection of the supernatant fluids of kidney cell homogenates into baby hamsters induced tumor growth similar to that induced by the polyomavirus.8 She realized the importance of her observation with regard to the polio vaccine used for mass vaccination. In the same year, during a lecture at the New York Cancer Society in which she discussed the tumorigenicity of polyomavirus, Eddy mentioned that she had found tumor-inducing viral agents in monkey kidney cells used for vaccine preparation. However, the NIH authorities had not authorized the comment and were concerned that the observation might affect the widespread use of the polio vaccine. They reprimanded Eddy for her insubordination and later demoted her. She was denied access to her laboratory and animals, and her publication was delayed 2 years. However, her observation was duplicated by the master vaccine producer Maurice Hilleman, PhD (1919-2005), at Merck who found that the monkey kidney cultures were indeed contaminated by simian vacuolating virus 40 (SV40) of the polyomavirus group (Figure 5).
The emerging picture was that in the polio vaccine given to millions of children between 1955 and 1963 remained the SV40 contaminant, which survived the dilute formalin treatment. In 1961, scientists concluded that SV40 could infect humans and showed that the virus transformed human cells in vitro. The virus could be isolated from mesotheliomas and brain tumors. Subsequently, the FDA ordered that the polio vaccine must be free of SV40. For awhile, the vaccine was prepared in green monkey kidney cells free of the contaminant virus. Currently, the vaccine is prepared in human diploid cells.
Results of a large study conducted by the National Cancer Institute in 1998 showed no elevated cancer incidence in persons who had received the SV40-contaminated polio vaccine. Researchers who conducted a similar study in Sweden came to the same conclusion. Routine vaccination has resulted in almost complete eradication of polio worldwide. Eddy continued her work at the Division of Biological Standards. In 1955, she received an honorary Doctor of Science degree from Marietta College in Marietta, Ohio. In 1967, the US Department of Health, Education and Welfare awarded her the Superior Service Medal.
MMR VACCINE CONTROVERSY THAT SERIOUSLY HARMED CHILDREN
The triple vaccine against measles, mumps, and rubella (MMR) was developed in the late 1960s by Hilleman. It contained live viruses attenuated by serial passages in chicken embryo cells or human diploid cells. After its marketing, 500 million doses were used in 60 countries. It is estimated that within the first 20 years of the vaccination, 52 million cases of measles were prevented and thousands of children were saved from mental retardation and death. Yet a single peer-reviewed study by Wakefield and colleagues9 that linked the triple vaccine, specifically the measles virus component, to intestinal inflammation and autism among children was enough to raise doubt about the vaccine’s safety that persists today. The 1998 study published in The Lancet was flawed because it examined only 12 vaccinated children of various ages without unvaccinated controls.
The publication had a huge impact on the parents of children who had been vaccinated. Fearful parents began refusing the vaccine. As a result, vaccination rates of babies in the United Kingdom declined from 95% in 1995 to 80% or less.10 Predictably, the incidence of measles rose. By 2008, almost 1400 cases were reported, with 2 confirmed deaths. A similar rise in measles cases occurred among unvaccinated children in Europe. Between 1998 and 2004, the medical community responded with numerous peer-reviewed publications in The Lancet, The New England Journal of Medicine, Pediatrics, and other journals that categorically refuted the spurious claim and showed no evidence between autism and the measles vaccine.
The Royal College of Physicians and the Medical Research Council also confirmed the safety of the vaccine. Epidemiological studies conducted by researchers in Denmark, Sweden, and Britain, as well as by committees of the Institute of Medicine and the National Academy of Sciences in the United States, further confirmed the absence of a link between measles vaccine and autism. The British General Medical Council found that Wakefield was guilty of professional misconduct, having subjected the children to unauthorized colonoscopy and lumbar puncture. In 2010, he was struck off the register and was prohibited to practice medicine in England. The Lancet retracted the publication. Wakefield is now in the United States, where he continues his activity to connect the measles vaccine to autism and has gained support from parents still suspicious of the vaccine and from Hollywood celebrities.
If the MMR vaccine–autism link was not scary enough, increasing awareness of the potential neurotoxicity of even low levels of organomercurial compounds in childhood vaccines caused a new uproar. Thimerosal (merthiolate), an ethylmercury compound, at 0.01% concentration was routinely added as a preservative to MMR, diphtheria, tetanus, pertussis, and other vaccines. It has been proven in animal experiments to be toxic by ingestion and inhalation. In a replay of the previous uproar, fearful parents in the United States and Europe again claimed that some children became autistic shortly after receiving the MMR vaccine with thimerosal and refused to have their babies vaccinated. Consequently, the incidence of measles outbreaks increased.
Epidemiological studies from Denmark, Sweden, and Britain found no link between the presence of the thimerosal and the occurrence of childhood autism. In 2004, the US Institute of Medicine, after having reviewed the published studies, concluded that no relationship could be found between the thimerosal in vaccines and autism. Yet the clamor continued, and vaccine producers finally stopped using the organomercurial compound as a preservative. An analysis on the prevalence of autism in California found that between 2004 and 2007, autism cases continued to rise even after thimerosal was withdrawn from the vaccines (see page S14 for a pro/con discussion on whether the topic of thimerosal-free vaccines should be discussed with parents).
References:
REFERENCES:
1. MaCrae T. Benjamin Jesty: a pre-Jennerianvaccinator. 1900; Vol 11, No S2390. Located at theWellcome Library, London.
2. Pead PJ. Benjamin Jesty: a new light on the dawnof vaccination. Lancet. 2003;362:2104-2109.
3. Fenner F, Henderson DA, Arita I, et al. Smallpoxand Its Eradication. Geneva: World Health Organization;1988.
4. Fine PE. Variation in protection by BCG: implicationsof and for heterologous immunity [publishedcorrection appears in Lancet. 1996;347:340]. Lancet.1995;346:1339-1345.
5. Dockrell HM, Zhang Y. A courageous step downthe road toward a new tuberculosis vaccine. Am JRespir Crit Care Med. 2009;179:628-629.
6. Lustbader ED, London WT, Blumberg BS. Studydesign for a hepatitis B vaccine trial. Proc Natl AcadSci U S A. 1976;73:955-959.
7. Francis T Jr, Korns RF, Voight RB, et al. Anevaluation of the 1954 poliomyelitis vaccine trials.Am J Public Health Nations Health. 1955;45(suppl,pt 2):1-63.
8. Eddy BE, Borman GS, Berkeley WH, Young RD.Tumors induced in hamsters by injection of rhesusmonkey kidney cell extracts. Proc Soc Exp Biol Med.1961;107:191-197.
9. Wakefield AJ, Murch SH, Anthony A, et al.Ileal-lymphoid-nodular hyperplasia, non-specificcolitis, and pervasive developmental disorder inchildren [retraction of Wakefield AJ, Murch SH,Anthony A, et al. In: Lancet. 2010;375:445]. Lancet.1998;351:637-417.
10. Thompson G. Measles and MMR statistics.House of Commons Library.
http://www.parliament.uk/briefingpapers/commons/lib/research/briefings/snsg-2581.pdf
. AccessedSeptember 23, 2010.