The latest Rx for sickle cell and oncology
A look at the most recent treatments for sickle cell disease and the latest pediatric oncology approvals.
Sickle cell disease
Sickle cell disease is the most common inherited blood disorder in the United States, affecting approximately 100,000 people. The causative mutation results in valine replacing glutamic acid as the 6th amino acid of the β-globin chain, making these red blood cells prone to sickling due to polymerization of deoxygenated hemoglobin S. However, the pathophysiology of sickle cell disease is more complex than red blood cell sickling, and includes increased expression of adhesion molecules on the vascular endothelium, leading to leukocyte adhesion and vasculopathy.1 Life expectancy of patients with sickle cell disease has improved from 28 years to 43 years since 1979 but remains shorter than the life expectancy for the general population.2 Neurologic complications, recurrent acute pain episodes, chronic pain, and acute chest syndrome are major causes of morbidity and mortality in this population.3
Role for prevention
Hydroxyurea has historically been the preventive strategy to decrease the occurrence of vaso-occlusive pain crises (VOC). VOC have been indicated to be responsible for more than 90% of hospital admissions in patients with sickle cell disease.4 Hematopoietic stem cell transplant is the curative treatment for sickle cell disease but major barriers prohibit its use, such as the limited availability of HLA-matched donors.5 This makes new strategies crucial to improving life expectancy and quality of life of patients with this disease.
Crizanlizumab
The US Food and Drug Administration (FDA) recently approved crizanlizumab to reduce the frequency of VOC in pediatric patients aged 16 years and older with sickle cell disease.6 Crizanlizumab is a humanized monoclonal antibody that binds to P-selectin and blocks its interaction with P-selectin glycoprotein ligand 1 (PSGL-1). Binding P-selectin on the activated endothelium and platelets blocks interactions between endothelial cells, platelets, red blood cells, and leukocytes that would eventually lead to vascular obstruction, tissue ischemia, and vaso-occlusion.6
The phase 2 SUSTAIN trial (NCT01895361) determined the efficacy of crizanlizumab in reducing the annual rate of sickle cell–related pain crises in 198 study participants.6 The study participants had experienced 2 to 10 sickle cell–related VOC in the 12 months prior to enrollment. They were assigned to receive low-dose crizanlizumab (2.5 mg/kg), high-dose crizanlizumab (5 mg/kg), or placebo over a study period of 52 weeks. The use of hydroxyurea was not an inclusion or exclusion criterion (participants receiving hydroxyurea were receiving it for at least 6 months, with no dose alterations.6)
According to the study results, the median rate of crises per year with high-dose crizanlizumab was 1.63 versus 2.98 with placebo, indicating a 45.3% lower rate of VOC with high-dose crizanlizumab (P = .01). The median time to the first crisis also was longer with high-dose crizanlizumab versus placebo, at 4.07 versus 1.38 months, respectively (P = .001). The primary outcomes in participants receiving the low-dose crizanlizumab were not found to be statistically significant. Adverse events from the study include arthralgia, diarrhea, pruritus, vomiting, and chest pain.6 The dose recommendation for crizanlizumab is 5 mg/kg intravenous (IV) on week 0, week 2, and every 4 weeks thereafter.7 Patients should be monitored for infusion-related reactions. No dose adjustments are required for hepatic or renal impairment.7 Further studies evaluating crizanlizumab in pediatric patients aged younger than 16 years are ongoing.
Voxelotor
The FDA also recently approved Voxelotor, which has a role in modifying the severity of disease through its unique mechanism as a hemoglobin modulator.8 Voxelotor acts as a polymerization inhibitor for hemoglobin S by reversibly binding hemoglobin to stabilize it in the oxygenated state.8
The international GBT_HOPE trial (NCT03036813), a phase 3, placebo-controlled study, evaluated the hemoglobin response to voxelotor.9 This study included 274 participants with sickle cell disease aged from 12 to 65 years; they had a hemoglobin level between 5.5 and 10.5 g/dL during screening and had experienced 1 to 10 VOC in the past 12 months. Participants were randomly assigned in a 1:1:1 ratio to daily oral dosing of 1500 mg, 900 mg, or placebo for a treatment period of up to 72 weeks. The majority were also receiving hydroxyurea that had been maintained at a stable dose for the past 3 months.9
A hemoglobin response, defined as an increase from baseline of more than 1 g/dL at week 24, was significantly greater in the 1500 mg voxelotor group (51% [46 of 90]; 95% CI, 41-61) compared with the placebo group (7% [6 of 92]; 95% CI, 1-12) in the intention-to-treat analysis (P < .001). The hemoglobin response was higher in the 1500 mg voxelotor group versus placebo regardless of concurrent hydroxyurea use or severity of anemia at baseline. Primary outcome in the 900 mg voxelotor group was not found to be statistically significant. The annualized adjusted incidence rate of VOC per person-year (95% CI) was 2.77 (2.15-3.57) in the 1500 mg group, 2.76 (2.15-3.53) in the 900 mg group, and 3.19 (2.5-4.07) in the placebo group, demonstrating a lower incidence of VOC in those receiving voxelotor.9
Adverse effects were similar for the 3 groups, with headache, diarrhea, and nausea common.9 The dose recommendation for voxelotor is 1500 mg daily in patients with sickle cell disease who are aged 12 years and older.8 Voxelotor is available as a 500-mg tablet. Dose adjustments are necessary with severe hepatic impairment and with concurrent use of interacting drugs (ie, CYP3A4 inducers and inhibitors).8
On the Horizon
These 2 new therapies offer pharmacotherapeutic options to prevent sickle cell–related complications, specifically VOC. Each one utilizes a different mechanism and offers the advantage of being able to be used concurrently with hydroxyurea. Looking to the future, early results from an ongoing clinical trial show that CRISPR-Cas9 gene editing has the potential to eliminate transfusion dependence and VOC episodes in patients with sickle cell disease.10 This has been demonstrated in 1 patient with sickle cell disease over a period of 16 months post treatment, and additional patients have more recently been enrolled in the study.10
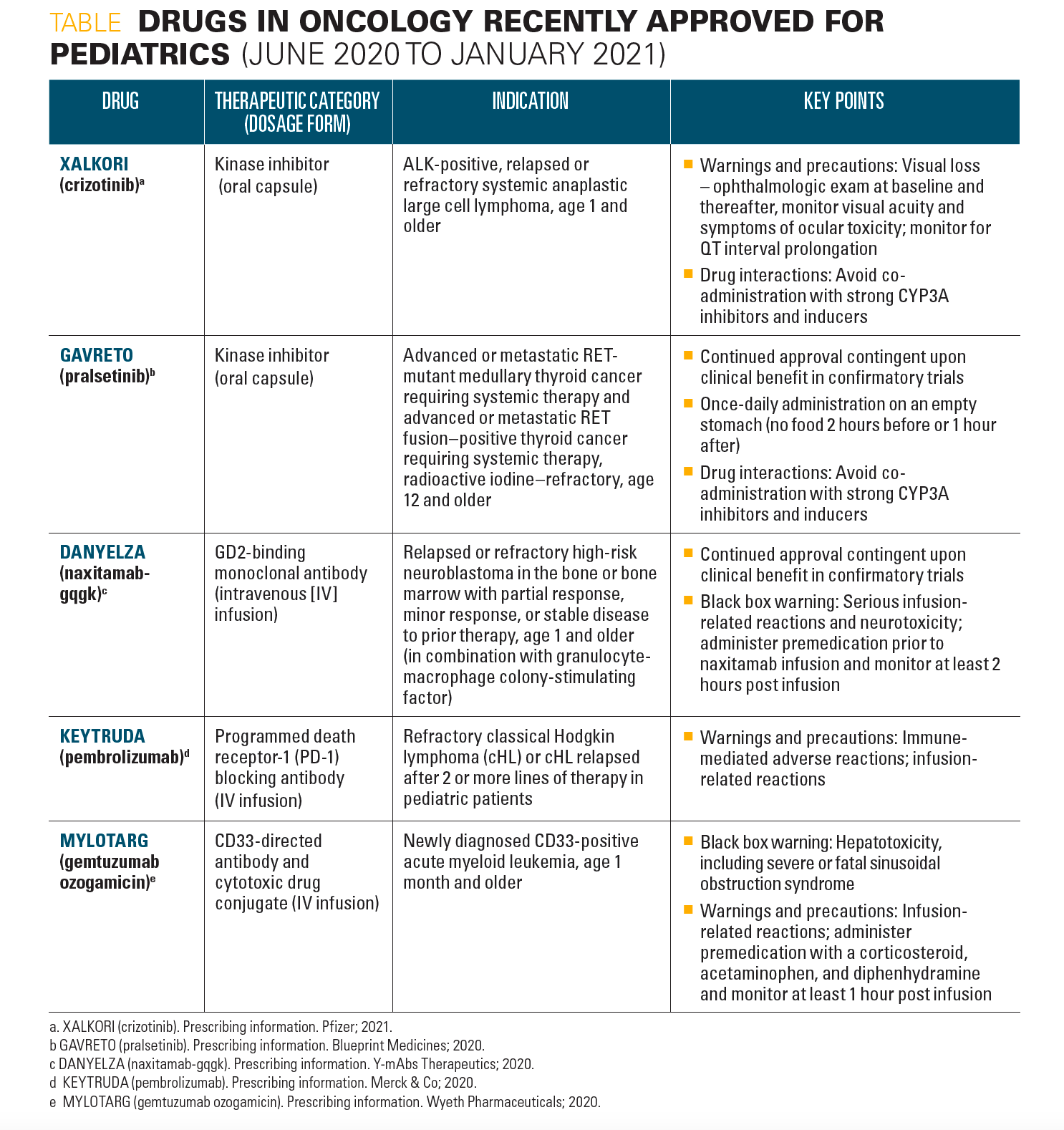
In addition to recent treatment advances in pediatric hematology, new drugs in oncology have been approved for use in pediatric patients from June 2020 to January 2021, as highlighted in the Table.
Table
References
1. Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11(2):129-151.
2. Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639-1644. doi:10.1056/NEJM199406093302303
3. Patterson GD, Mashegu H, Rutherford J, et al. Recurrent acute chest syndrome in pediatric sickle cell disease: clinical features and risk factors. J Pediatr Hematol Oncol. 2018;40(1):51-55. doi:10.1097/MPH.0000000000001012
4. Sins JWR, Mager DJ, Davis SCA, Biemond BJ, Fijnvandraat K. Pharmacotherapeutical strategies in the prevention of acute, vaso-occlusive pain in sickle cell disease: a systematic review. Blood Adv. 2017;1(19):1598-1616. doi:10.1182/bloodadvances.2017007211
5. Angelucci E, Matthes-Martin S, Baronciani D, et al; EBMT Inborn Error and EBMT Paediatric Working Parties. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica. 2014;99(5):811-820. doi:10.3324/haematol.2013.099747
6. Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429-439. doi:10.1056/NEJMoa1611770
7. Adakveo. Prescribing information. Novartis Pharmaceuticals Corporation; 2019.
8. Oxbryta. Prescribing information. Global Blood Therapeutics, Inc; 2019.
9. Vichinsky E, Hoppe CC, Ataga KI, et al; HOPE Trial Investigators. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381(6):509-519. doi:10.1056/NEJMoa1903212
10. Frangoul H, Altshuler D, Cappellini MD, et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N Engl J Med. 2021;384(3):252-260. doi:10.1056/NEJMoa2031054

Having "the talk" with teen patients
June 17th 2022A visit with a pediatric clinician is an ideal time to ensure that a teenager knows the correct information, has the opportunity to make certain contraceptive choices, and instill the knowledge that the pediatric office is a safe place to come for help.
Meet the Board: Vivian P. Hernandez-Trujillo, MD, FAAP, FAAAAI, FACAAI
May 20th 2022Contemporary Pediatrics sat down with one of our newest editorial advisory board members: Vivian P. Hernandez-Trujillo, MD, FAAP, FAAAAI, FACAAI to discuss what led to her career in medicine and what she thinks the future holds for pediatrics.