Aplasia Cutis Congenita With Associated Fetus Papyraceus
A baby boy born after normal vaginal delivery at 36 weeks’ gestation was noted to have a distinct abdominal wall lesion. Apgar scores were 8 and 9 at 1 and 5 minutes, respectively.
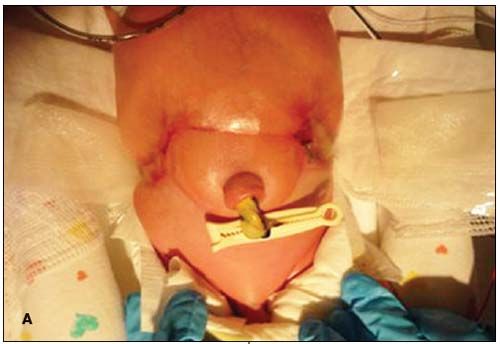
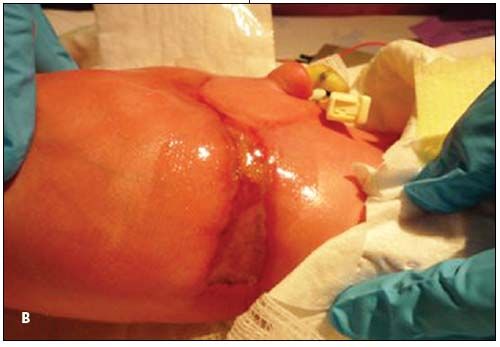
A baby boy born after normal vaginal delivery at 36 weeks’ gestation was noted to have a distinct abdominal wall lesion. Apgar scores were 8 and 9 at 1 and 5 minutes, respectively. Weight was 2300 g. The infant appeared well-nourished and nondysmorphic. The ulcerated, erythematous-violaceous, bandlike lesion encircled the umbilical stump (A), involved both flanks (B), and appeared well-demarcated and constrictive. The infant’s posterior trunk and umbilical cord were normal. Examination of the placental membranes revealed the mummified remains of a twin fetus with an umbilical cord of 80 mm. An ultrasonogram at 10 weeks’ gestation had demonstrated the demise of the twin fetus. There was no family history of congenital defects. The mother denied teratogenic exposures during pregnancy.
Aplasia cutis congenita (ACC) with associated fetus papyraceus was diagnosed. About 500 cases of ACC have been reported, 45 of which have been associated with fetus papyraceus. ACC is characterized by multiple congenital skin defects, 84% of which are located on the scalp.1 In Frieden’s2 proposed clinical classification of ACC, ACC with associated fetus papyraceus or placental infarcts usually presents as symmetric, linear, stellate areas of aplasia-the distribution pattern characteristically forms an “H” on the trunk.3 Associated clinical findings include spastic paralysis, mental retardation, hydranencephaly, nail dystrophy, and clubbed extremities.2 The lesions appear to be sporadic, with no increased risk in subsequent pregnancies.
Fetus papyraceus usually occurs with intrauterine death of the twin during the late first to early second trimester, whereas an earlier or later death typically leads to complete resorption or maceration, respectively.1 Although controversial, the major cause of dermal ischemia after the death of a twin may be rapid exsanguination of the surviving twin. Relaxation of the circulation in the dying twin is followed by acute hypovolemia of the surviving twin. This is referred to as the “hemodynamic theory” and may explain the characteristic dermal pattern of ACC, which reflects “watershed” areas.3,4 The tissue furthest from the arterial origin is at greatest risk for ischemic lesions during episodes of hypovolemia.3 Factors that support this hypothesis include fetal blood sampling that documents anemia but normal coagulation profiles in the surviving twin5 and Doppler ultrasonography that detects acute transfusion from the surviving twin to the dying twin.6
The primary treatment goal is prevention of fatal complications, such as bleeding, sepsis, and possible traumatic perforation or peritonitis secondary to bacterial invasion of large skin and muscle defects of the abdominal region.7 Management may be conservative, surgical, or a combination of both. Large defects may require skin grafting or even local flaps. Less severe defects may be treated with topical silver sulfadiazine or mupirocin and an antibacterial dressing that resists scar drying and separation and allows epithelialization.8
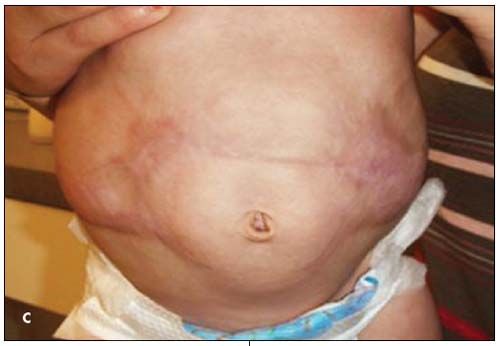
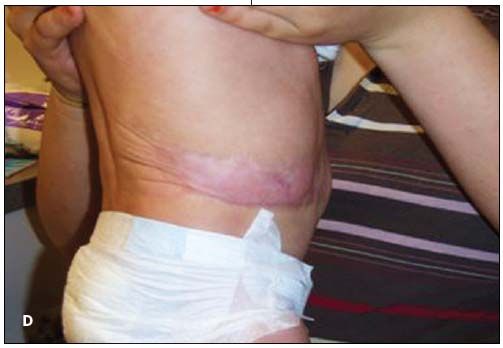
This infant was treated with a 7-day course of parenteral antibiotics for suspected sepsis. The lesion was managed conservatively with petroleum gauze dressings, changed twice daily, alternated with sterile water debridement during dressing changes. After 7 days, early epithelialization had occurred, and the infant was discharged with local wound care instructions and dermatology follow-up. At 3 months, the lesions had granulated (C and D). He had no restriction of growth or mobility and no internal organ involvement or dysfunction. Scar revision is a potential future cosmetic intervention. It is anticipated that he will have normal long-term growth and development.
References:
REFERENCES:
1. Mannino FL, Jones KL, Benirschke K. Congenital skin defects and fetus papyraceus. J Pediatr. 1977; 91:559-564.
2. Frieden IJ. Aplasia cutis congenita: a clinical review and proposal for classification. J Am Acad Dermatol. 1986;14:646-660.
3. Schaffer JV, Popiolek DA, Orlow SJ. Symmetric truncal aplasia cutis congenita following multifetal reduction of a sextuplet pregnancy. J Pediatr. 2008;153:860-863.
4. Lewi L, Van Schoubroeck D, Gratacós E, et al. Monochorionic diamniotic twins: complications and management options. Curr Opin Obstet Gynecol. 2003;15:177-194.
5. Nicolini U, Pisoni MP, Cela E, Roberts A. Fetal blood sampling immediately before and within 24 hours of death in monochorionic twin pregnancies complicated by single intrauterine death. Am J Obstet Gynecol. 1998;179(3, pt 1):800-803.
6. Gembruch U, Viski S, Bagamery K, et al. Twin reversed arterial perfusion sequence in twin-to-twin transfusion syndrome after the death of the donor co-twin in the second trimester. Ultrasound Obstet Gynecol. 2001;17:153-156.
7. Verhelle NA, Heymans O, Deleuze JP, et al. Abdominal aplasia cutis congenita: case report and review of the literature. J Pediatr Surg. 2004;39: 237-239.
8. Ross DA, Laurie SW, Coombs CJ, Mutimer KL. Aplasia cutis congenita: failed conservative treatment. Plast Reconstr Surg. 1995;95:124-129.