Breath-actuated inhalers in childhood asthma
Tailoring drug delivery modalities to the individual patient based on age, ability level, and preference can optimize control of pediatric asthma.
Carlton Lee, PharmD, MPH, FASHP, FPPAG

Nicholas A Jabre, MD, MS

headshot of Mandeep Jassal, MD, MPH

Introduction to Pharmacologist's notebook

Table
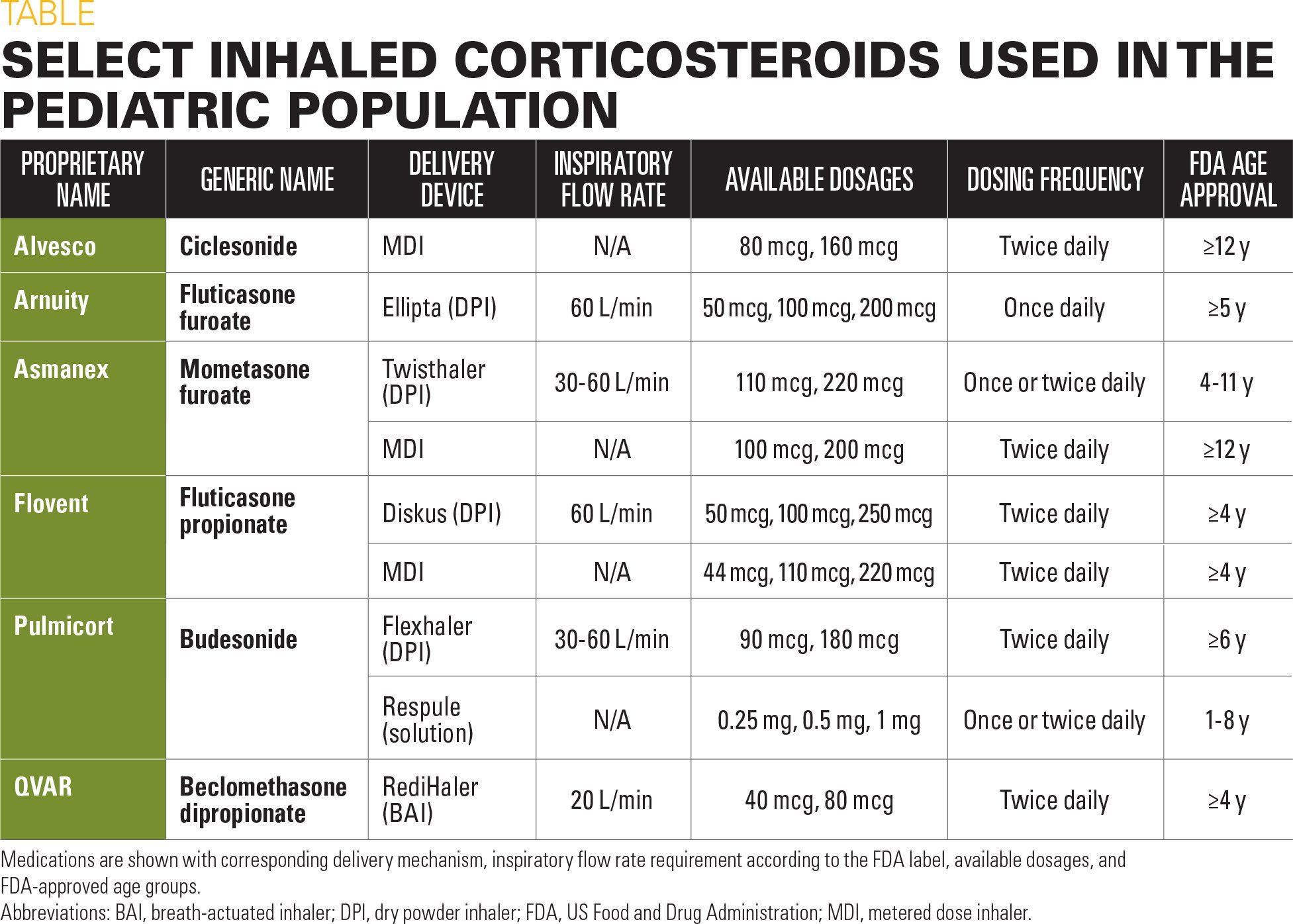
Asthma medication for children

In February 2018, the pharmaceutical company Teva (Petah Tikva, Israel) replaced its QVAR (beclomethasone dipropionate) pressurized metered-dose inhaler (pMDI) with a breath-actuated inhaler (BAI) known as the RediHaler. Drug delivery of inhaled corticosteroids has traditionally been accomplished using a pMDI. However, some patients have difficulty synchronizing device actuation with inhalation. The BAI was developed to overcome hand-breath coordination problems and utilizes the patient’s inspiratory flow to trigger release of an aerosol.1
Although theoretically easier to use than a pMDI, the BAI is not used with a spacer and requires a sustained inspiratory effort that may be difficult for young children to perform.2 This article discusses the use of BAIs in children and poses alternative treatments for those who cannot perform the inhalation maneuvers required for use of this device.
Advantages of BAIs
In a randomized trial, BAIs were shown to be therapeutically equivalent to pMDIs, assuming appropriate pMDI technique.3 However, a BAI device achieves improved drug deposition in the lungs compared with a pMDI device that is used with poor hand-breath coordination.2,4 The BAI also has increased ease of use, with 55% of subjects in 1 study reporting it to be “extremely easy,” compared with only 41% in the pMDI group.3
Additionally, the BAI is triggered by a relatively low flow rate compared with currently available dry powder inhalers (DPIs) and may be considered a good choice for children who cannot generate powerful inspiratory flows. It is a valuable delivery device and should be considered for pediatric patients who meet the criteria for its usage.
Disadvantages of BAIs
There are several important limitations of the BAI device. A flow rate below the activating threshold will not trigger the dose-delivery mechanism, which precludes drug release.5 Thus, a BAI is not appropriate for infants and children who cannot perform a sustained inhalation of sufficient magnitude or whose only possible inhalation technique is restful breathing. Among children who can trigger the device, those aged 5 to 7 years appear to receive less drug deposition into the lungs and more into the oropharynx compared with older groups.6 Another drawback of the BAI device is the requirement of breath holding after inhalation. A study examining the effect of various breath hold times on inhaled corticosteroid deposition in the lungs found a 16% reduction following a 1-second versus a 10-second breath hold time.7
Other delivery systems
A summary of currently available inhaled corticosteroids and corresponding delivery devices is provided in the Table. Each delivery device has its own set of advantages and limitations, which should be carefully considered before treatment.
Similar to BAIs, DPIs such as the Arnuity Ellipta can overcome hand-breath coordination problems. However, the delivered dose is flow- and acceleration-dependent, such that suboptimal inhalation velocity leads to larger particle size and more deposition in the mouth and oropharynx.8 Children may also be unable to generate adequate inspiratory flow during periods of acute wheezing.2 Respiratory trainers are available to determine if patients can generate sufficient flow for specific devices.9
Nebulized treatments likewise eliminate the need for coordinated breathing but result in nonuniform particle sizes and drug loss to the environment. They additionally require a power source and long treatment times, which may impose an unnecessary burden on patients and their families.10
Guidelines for inhaler selection
To assist the practitioner, the Global Initiative for Asthma (GINA), other expert consensus panels, and authorities in the field have proposed guidelines for inhaler selection in young children. Breath-actuated devices should only be used in children aged 6 to 7 years and older, as the inspiration time in patients aged younger than this is generally too short to complete an effective inhalation.2,10 A pMDI with a valved holding chamber is the preferred delivery system for patients aged 5 years and younger. The spacer should be used with a face mask in children aged 0 to 3 years because restful breathing is the only possible inhalation method in this group. Nebulizers should be reserved for the minority of these patients who cannot be trained to effectively use a spacer.11
Once an effective delivery mechanism has been identified, it should not be switched without a clinical visit or consultation, because this has been associated with worsened asthma control.12
Summary
Staying informed about the advantages and limitations of currently available inhaler devices, including the new QVAR RediHaler, is an essential duty of asthma practitioners and pharmacy benefits management-plan providers alike. Ultimately, tailoring drug delivery modalities to the individual patient based on age, ability level, and preference can optimize disease control and should be highly encouraged.
References:
1. Stein SW, Thiel CG. The history of therapeutic aerosols: a chronological review. J Aerosol Med Pulm Drug Deliv. 2017;30(1):20-41.
2. van Aalderen WM, Garcia-Marcos L, Gappa M, et al. How to match the optimal currently available inhaler device to an individual child with asthma or recurrent wheeze. NPJ Prim Care Respir Med. 2015;25:14088.
3. Murphy KR, Dhand R, Trudo F, Uryniak T, Aggarwal A, Eckerwall G. Therapeutic equivalence of budesonide/formoterol delivered via breath-actuated inhaler vs pMDI. Respir Med. 2015;109(2):170-179.
4. Leach CL, Davidson PJ, Hasselquist BF, Boudreau RJ. Influence of particle size and patient dosing technique on lung deposition of HFA-beclomethasone from a metered dose inhaler. J Aerosol Med. 2005;18(4):379-385.
5. Haidl P, Heindl S, Siemon K, Bernacka M, Cloes RM. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75.
6. Devadason SG, Huang T, Walker S, Troedson R, Le Souëf PN. Distribution of technetium-99m-labelled QVAR delivered using an Autohaler device in children. Eur Respir J. 2003;21(6):1007-1011.
7. Leach CL, Colice GL. A pilot study to assess lung deposition of HFA-beclomethasone and CFC-beclomethasone from a pressurized metered dose inhaler with and without add-on spacers and using varying breath-hold times. J Aerosol Med Pulm Drug Deliv. 2010;23(6):355-361.
8. Virchow JC, Crompton GK, Dal Negro R, et al. Importance of inhaler devices in the management of airway disease. Respir Med. 2008;102(1):10-19.
9. Bossard MK. In-check DIAL inspiratory trainer. J Asthma Allergy Educators. 2010;1(4):160-161.
10. Laube BL, Janssens HM, de Jongh FH, et al; European Respiratory Society; International Society for Aerosols in Medicine. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308–1331.
11. Global Initiative for Asthma. 2018 GINA Report, Global Strategy for Asthma Management and Prevention. Available at: https://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/. Published 2018. Accessed August 20, 2018.
12. Thomas M, Price D, Chrystyn H, Lloyd A, Williams AE, von Ziegenweidt J. Inhaled corticosteroids for asthma: impact of practice level device switching on asthma control. BMC Pulm Med. 2009;9:1.

The Role of the Healthcare Provider Community in Increasing Public Awareness of RSV in All Infants
April 2nd 2022Scott Kober sits down with Dr. Joseph Domachowske, Professor of Pediatrics, Professor of Microbiology and Immunology, and Director of the Global Maternal-Child and Pediatric Health Program at the SUNY Upstate Medical University.
Overview of biologic drugs in children and adolescents
March 10th 2025A presentation at the 46th National Association of Pediatric Nurse Practitioners (NAPNAP) conference explored the role of biologics in pediatric care, their applications in various conditions, and safety considerations for clinicians.