Conference Coverage
3 months ago
Our coverage recap from IDWeek 202511 months ago
Measles outbreaks: 223 cases reported in Texas11 months ago
NAPNAP conference provides voice for pediatric careover 1 year ago
Understanding TMS therapy for major depressive disorderover 1 year ago
Clesrovimab: A potential new player to protect against RSVover 1 year ago
Nonoperative treatment emerging for pediatric appendicitisover 1 year ago
Pertussis rates continue to rise in the United Statesover 1 year ago
Study finds parents open to firearms discussion from doctorsover 1 year ago
'Chroming' trend among teens linked to TikTok videosover 1 year ago
Increasing child opioid overdose casesover 1 year ago
Low vitamin D levels associated with slowed fracture healingover 1 year ago
Itchy skin associated with sleep problems in infantsalmost 2 years ago
Effect of Goals-Activity-Motor Enrichment in infants with cerebral palsyalmost 2 years ago
Managing and treating eating disordersalmost 2 years ago
Patient-centered approach to updated AAP breastfeeding guidelinesalmost 2 years ago
Incorporating PrEP and PEP into school-based health centersalmost 2 years ago
Severe refractory status asthmaticus, research, and current trendsalmost 2 years ago
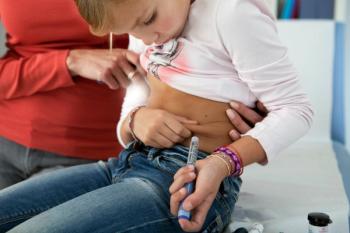
Primary care digital innovations in type 1 diabetesalmost 2 years ago
Screening for perinatal mental health disordersalmost 2 years ago
Congenital heart disease and associated genetic red flagsalmost 2 years ago
Creating mobile mental health clinics to improve access for rural youthalmost 2 years ago
Mobile Autism Screening Tool Could Improve Primary Care Diagnosesalmost 2 years ago
Secondhand vape exposure detectable in children's saliva, breathover 2 years ago
Implementing early autism screening into your practiceover 2 years ago
Negotiating to get paid what you deserveover 2 years ago
Young athletes, social media, and body image issuesover 2 years ago
Delta-8 accessibility to minors onlineover 2 years ago
How childhood sexual abuse affects adult survivorsover 2 years ago
RSV: 1 year post-tripledemic emergency department updateover 2 years ago
Update on allergy prevention and food introductionover 2 years ago
Evaluating risk of suicide and limiting youth access to firearmsover 2 years ago
High-powered magnet ingestion and socioeconomic disparitiesover 2 years ago
Preterm infant HRQOL: Long-term impacts and determinantsover 2 years ago
Safe sleep practices among parentsover 2 years ago
What’s happening in the exhibition hall at the AAP conferenceover 2 years ago
Nature exposure therapy for pediatric patients with chronic painover 2 years ago
AAP National Conference & Exhibition: what you can expectover 2 years ago
What’s new for maternal Group B Streptococcus vaccines?over 2 years ago
Genital herpes: expanding the toolboxover 2 years ago
The transformative impact of HPV vaccinesover 2 years ago
Understanding post-viral sequelae through the lens of HIVover 2 years ago
Pediatric immunocompromised hosts: a glimpse into the futureover 2 years ago
TB in pediatric patients is not so weeover 2 years ago
Should neonates with bacteremic UTI be treated with IV only?over 2 years ago
Mpox: where we were, where we are nowalmost 3 years ago
How air pollution and climate change impact infants and birthing peoplealmost 3 years ago
The fallout from COVID-19 for children and familiesalmost 3 years ago
Incorporating the Rapid Interactive Screen Test for ASD in primary carealmost 3 years ago
How to support the breastfeeding parentalmost 3 years ago
How will artificial intelligence change your pediatric practice?almost 3 years ago
Steven Selbst, MD, previews his physician burnout research at PAS 2023almost 3 years ago
Highlighting pediatric nurse practitionersalmost 3 years ago
How to manage viral infections in pediatric patientsalmost 3 years ago
CDC immunization schedule updatesalmost 3 years ago
Managing disordered eating in adolescents with type 1 diabetesalmost 3 years ago
Diagnosing and treating PCOS in adolescentsover 3 years ago
AAP reports on which children are at greatest risk for COVID-19over 3 years ago
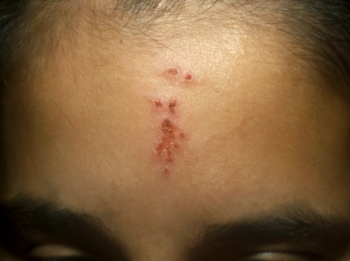
Skin eruptions on infants: self-resolving or concerning?over 3 years ago
Preventing childhood injury in the homeover 3 years ago
Booster seats in a bag: A pandemic successover 3 years ago
Telemedicine intervention effective against childhood obesityover 3 years ago
Practical strategies to help the picky eaterover 3 years ago
What is relational health, and why is it so important?over 3 years ago
Highlights from the AAP conferenceover 3 years ago
Let's get excited about the AAP Conference!over 3 years ago
A talk with Albert Yan, MD on current trends in technologyover 3 years ago
Telederm: friend or foeover 3 years ago
COVID-19: a look back and a look forwardover 3 years ago
Acne: therapeutic updates and our most challenging casesover 3 years ago
Skin picking and other habitual disordersover 3 years ago
Hot topics at the SPD 2022 47th Annual Meetingalmost 4 years ago
Do preschool children with ADHD get the behavioral therapy they need?almost 4 years ago
The effect of parental food access on high birthweightalmost 4 years ago
Are you a 5-star pediatrician?almost 4 years ago
Adolescents, mental health, and TikTok usealmost 4 years ago
Celiac disease and the gluten-free dietalmost 4 years ago
Integrative approaches to common GI disordersalmost 4 years ago
Exhausted and burned out: results of a survey of nurse practitionersalmost 4 years ago
Immunization updates for 2022almost 4 years ago
Newest medications for ADHDalmost 4 years ago
How to tell if your patient has an eating disorderalmost 4 years ago
Prescribing antibiotics for kids in 2022almost 4 years ago
What to look forward to at NAPNAP 2022over 4 years ago
Reaching teens on sexual health through telehealthover 4 years ago
Examining when implicit biases influence clinical careover 4 years ago
Using a lifestyle approach to manage adolescent depressionover 4 years ago
Medication nonadherence: What may cause it and how to prevent itover 4 years ago
Pearls for treating dermatologic conditions in skin of colorover 4 years ago
Essential advice to prevent food allergiesover 4 years ago
Tips for accurately coding telehealth servicesover 4 years ago
Diagnosing and managing menstrual disorders in teen girlsover 4 years ago
Basic epidemiology for pediatriciansover 4 years ago
Removing barriers to care for immigrant kidsover 4 years ago
Advanced coping skills for pediatricians during high stressover 4 years ago
What's new for atopic dermatitis in childrenover 4 years ago
Diagnosing and treating neonatal hypoglycemiaover 4 years ago
What are the long-term outcomes of neonatal hypoglycemia?over 4 years ago
The future of pediatric type 2 diabetesover 4 years ago
Epidemiology of type 2 diabetes–SEARCH updateover 4 years ago
Can food insecurity lead to poorer type 2 diabetes outcomes?over 4 years ago
Vaccine Roundtable at the World Vaccine Congress Washington 2021almost 5 years ago
Highlights from the 30th Pediatric Pharmacy Association Meetingalmost 5 years ago
Improve discharge safety with a checklistalmost 5 years ago
Using ED visits to provide counsel on contraceptivesalmost 5 years ago
Non-adherence to treatment is multifactorialalmost 5 years ago
Impact of the pandemic on kids with autism is significantalmost 5 years ago
Using an algorithm to be an upstander in the face of discriminationalmost 5 years ago
Examining the efficacy of cord clamp delayabout 5 years ago
Conference Club: 6 in-depth stories from AAPabout 5 years ago
Quick hits from AAP 2020about 5 years ago
Optimizing antibiotic use and infectious diagnostic testingabout 5 years ago
Psychopharmacology for primary care providersabout 5 years ago
Updates on pediatric dermatologyabout 5 years ago
Protecting kids with autism from harm due to wanderingover 5 years ago
Dr Anthony Fauci talks COVID-19 at AAP conferenceover 16 years ago
Honors for 4 Pediatric Heroesover 16 years ago
Health Care Reform: What’s in It for Kids?over 16 years ago
Sessions You Don’t Want to Miss at the AAP NCELatest News

Weekly review: diabetes screening, assisted delivery outcomes, and more

Neurodevelopmental disorders common after infant heart surgery

High-dose therapy boosts arm function in infants after perinatal stroke

Renuka Dias, PhD, MBBS, highlights feasibility of type 1 diabetes screening in children

FDA grants priority review to marstacimab (Hympavzi) for younger children and patients with hemophilia inhibitors

Shorts










Podcasts
Videos
Contemporary Pediatrics Digital Edition








Continuing Medical Education
All News

A recent study found that nearly half of parents of children with high weight reported concerns about disordered eating.

FDA and partners are investigating a multistate infant botulism outbreak linked to powdered formula and assessing risks of Clostridium botulinum.

Giulia M. Muraca, PhD, MPH, and Maya Rajasingham, BSc, discuss new data comparing neurodevelopmental outcomes in children born via second-stage cesarean and assisted vaginal delivery.

A study shows how COVID-19 reshaped the circulation, seasonality, and coinfection patterns of respiratory viruses in children.

Experts urge culturally responsive nutrition counseling, noting the new Dietary Guidelines lack guidance on diverse food traditions and eating patterns.

Experts discuss access, affordability, and equity challenges in applying the new Dietary Guidelines, urging clinicians to reduce guilt and personalize care.

Experts debate stricter added sugar recommendations in the new Dietary Guidelines, stressing practicality, age-specific needs, and personalized counseling.

Experts flag missing pediatric guidance in the 2025–2030 Dietary Guidelines, including infant safety, iron intake, and early nutrition education.

Experts highlight gaps in the new Dietary Guidelines, including fiber, whole grains, plant-based milks, and simplified alcohol guidance.

Experts unpack saturated fat recommendations in the 2025–2030 Dietary Guidelines, highlighting visual misalignment and the need for personalized counseling.

Experts clarify protein recommendations in the 2025–2030 Dietary Guidelines, emphasizing quality, preparation, and clinician-led patient education.

Experts discuss whole-foods emphasis, microbiome insights, and consumer-focused changes in the 2025–2030 US Dietary Guidelines for clinicians.

Take a quick look at everything you may have missed this past month, including the top FDA approvals and latest clinical updates.

A look back at the FDA submissions and regulatory decisions in the pediatric health care space from January 2026.

Deep transfer learning models may enable more accurate, globally applicable prediction of spoken language outcomes in deaf children receiving cochlear implants.